High Salinity Induces Different Oxidative Stress and Antioxidant Responses in Maize Seedlings Organs
- PMID: 27014300
- PMCID: PMC4781871
- DOI: 10.3389/fpls.2016.00276
High Salinity Induces Different Oxidative Stress and Antioxidant Responses in Maize Seedlings Organs
Abstract
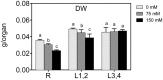
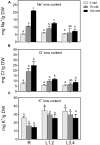
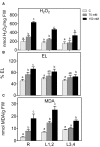
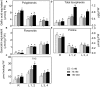
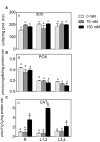
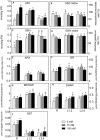
Salinity negatively affects plant growth and causes significant crop yield losses world-wide. Maize is an economically important cereal crop affected by high salinity. In this study, maize seedlings were subjected to 75 mM and 150 mM NaCl, to emulate high soil salinity. Roots, mature leaves (basal leaf-pair 1,2) and young leaves (distal leaf-pair 3,4) were harvested after 3 weeks of sowing. Roots showed the highest reduction in biomass, followed by mature and young leaves in the salt-stressed plants. Concomitant with the pattern of growth reduction, roots accumulated the highest levels of Na(+) followed by mature and young leaves. High salinity induced oxidative stress in the roots and mature leaves, but to a lesser extent in younger leaves. The younger leaves showed increased electrolyte leakage (EL), malondialdehyde (MDA), and hydrogen peroxide (H2O2) concentrations only at 150 mM NaCl. Total antioxidant capacity (TAC) and polyphenol content increased with the increase in salinity levels in roots and mature leaves, but showed no changes in the young leaves. Under salinity stress, reduced ascorbate (ASC) and glutathione (GSH) content increased in roots, while total tocopherol levels increased specifically in the shoot tissues. Similarly, redox changes estimated by the ratio of redox couples (ASC/total ascorbate and GSH/total glutathione) showed significant decreases in the roots. Activities of enzymatic antioxidants, catalase (CAT, EC 1.11.1.6) and dehydroascorbate reductase (DHAR, EC 1.8.5.1), increased in all organs of salt-treated plants, while superoxide dismutase (SOD, EC 1.15.1.1), ascorbate peroxidase (APX, EC 1.11.1.11), glutathione-s-transferase (GST, EC 2.5.1.18) and glutathione reductase (GR, EC 1.6.4.2) increased specifically in the roots. Overall, these results suggest that Na(+) is retained and detoxified mainly in roots, and less stress impact is observed in mature and younger leaves. This study also indicates a possible role of ROS in the systemic signaling from roots to leaves, allowing leaves to activate their defense mechanisms for better protection against salt stress.
Keywords: antioxidants; biomass; maize; oxidative stress; reactive oxygen species (ROS); salinity.
Figures
References
-
- Aebi H. (1984). “Catalase in vitro,” in Methods in Enzymology ed. Lester P. (Cambridge, MA: Academic Press; ) 121–126. - PubMed
-
- Ambede J. G., Netondo G. W., Mwai G. N., Musyimi D. M. (2012). NaCl salinity affects germination, growth, physiology, and biochemistry of bambara groundnut. Br. J. Plant Physiol. 24 151–160. 10.1590/S1677-04202012000300002 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

