Phytochrome A Mediates Blue-Light Enhancement of Second-Positive Phototropism in Arabidopsis
- PMID: 27014313
- PMCID: PMC4786545
- DOI: 10.3389/fpls.2016.00290
Phytochrome A Mediates Blue-Light Enhancement of Second-Positive Phototropism in Arabidopsis
Abstract
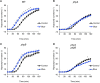
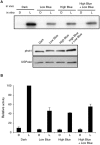
Hypocotyl phototropism of etiolated Arabidopsis seedlings is primarily mediated by the blue-light receptor kinase phototropin 1 (phot1). Phot1-mediated curvature to continuous unilateral blue light irradiation (0.5 μmol m(-2) s(-1)) is enhanced by overhead pre-treatment with red light (20 μmol m(-2) s(-1) for 15 min) through the action of phytochrome (phyA). Here, we show that pre-treatment with blue light is equally as effective in eliciting phototropic enhancement and is dependent on phyA. Although blue light pre-treatment was sufficient to activate early phot1 signaling events, phot1 autophosphorylation in vivo was not found to be saturated, as assessed by subsequently measuring phot1 kinase activity in vitro. However, enhancement effects by red and blue light pre-treatment were not observed at higher intensities of phototropic stimulation (10 μmol m(-2) s(-1)). Phototropic enhancement by red and blue light pre-treatments to 0.5 μmol m(-2) s(-1) unilateral blue light irradiation was also lacking in transgenic Arabidopsis where PHOT1 expression was restricted to the epidermis. Together, these findings indicate that phyA-mediated effects on phot1 signaling are restricted to low intensities of phototropic stimulation and originate from tissues other than the epidermis.
Keywords: blue light; epidermis; phosphorylation; phototropin; phototropism; phytochrome; red light.
Figures





Similar articles
-
A phytochrome/phototropin chimeric photoreceptor of fern functions as a blue/far-red light-dependent photoreceptor for phototropism in Arabidopsis.Plant J. 2015 Aug;83(3):480-8. doi: 10.1111/tpj.12903. Epub 2015 Jul 2. Plant J. 2015. PMID: 26095327
-
Hypocotyl growth orientation in blue light is determined by phytochrome A inhibition of gravitropism and phototropin promotion of phototropism.Plant J. 2004 Dec;40(5):826-34. doi: 10.1111/j.1365-313X.2004.02256.x. Plant J. 2004. PMID: 15546364
-
The enhancement of phototropin-induced phototropic curvature in Arabidopsis occurs via a photoreversible phytochrome A-dependent modulation of auxin responsiveness.Plant Physiol. 2001 Jun;126(2):826-34. doi: 10.1104/pp.126.2.826. Plant Physiol. 2001. PMID: 11402210 Free PMC article.
-
The action of enhancing weak light capture via phototropic growth and chloroplast movement in plants.Stress Biol. 2022 Dec 1;2(1):50. doi: 10.1007/s44154-022-00066-x. Stress Biol. 2022. PMID: 37676522 Free PMC article. Review.
-
Photosensory adaptation mechanisms in hypocotyl phototropism: how plants recognize the direction of a light source.J Exp Bot. 2023 Mar 28;74(6):1758-1769. doi: 10.1093/jxb/erad015. J Exp Bot. 2023. PMID: 36629282 Review.
Cited by
-
Response to comment on 'Lack of evidence for associative learning in pea plants'.Elife. 2020 Sep 10;9:e61689. doi: 10.7554/eLife.61689. Elife. 2020. PMID: 32909944 Free PMC article.
-
Influence of a phyA Mutation on Polyamine Metabolism in Arabidopsis Depends on Light Spectral Conditions.Plants (Basel). 2023 Apr 18;12(8):1689. doi: 10.3390/plants12081689. Plants (Basel). 2023. PMID: 37111912 Free PMC article.
-
Two distinct light-induced reactions are needed to promote germination in spores of Ceratopteris richardii.Front Plant Sci. 2023 Jun 2;14:1150199. doi: 10.3389/fpls.2023.1150199. eCollection 2023. Front Plant Sci. 2023. PMID: 37332704 Free PMC article.
-
Deetiolation Enhances Phototropism by Modulating NON-PHOTOTROPIC HYPOCOTYL3 Phosphorylation Status.Plant Physiol. 2019 Jun;180(2):1119-1131. doi: 10.1104/pp.19.00206. Epub 2019 Mar 27. Plant Physiol. 2019. PMID: 30918082 Free PMC article.
-
Functional characterization of Arabidopsis phototropin 1 in the hypocotyl apex.Plant J. 2016 Dec;88(6):907-920. doi: 10.1111/tpj.13313. Epub 2016 Oct 14. Plant J. 2016. PMID: 27545835 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

