Target of Rapamycin Is a Key Player for Auxin Signaling Transduction in Arabidopsis
- PMID: 27014314
- PMCID: PMC4786968
- DOI: 10.3389/fpls.2016.00291
Target of Rapamycin Is a Key Player for Auxin Signaling Transduction in Arabidopsis
Abstract
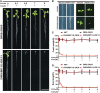
Target of rapamycin (TOR), a master sensor for growth factors and nutrition availability in eukaryotic species, is a specific target protein of rapamycin. Rapamycin inhibits TOR kinase activity viaFK506 binding protein 12 kDa (FKBP12) in all examined heterotrophic eukaryotic organisms. In Arabidopsis, several independent studies have shown that AtFKBP12 is non-functional under aerobic condition, but one study suggests that AtFKBP12 is functional during anaerobic growth. However, the functions of AtFKBP12 have never been examined in parallel under aerobic and anaerobic growth conditions so far. To this end, we cloned the FKBP12 gene of humans, yeast, and Arabidopsis, respectively. Transgenic plants were generated, and pharmacological examinations were performed in parallel with Arabidopsis under aerobic and anaerobic conditions. ScFKBP12 conferred plants with the strongest sensitivity to rapamycin, followed by HsFKBP12, whereas AtFKBP12 failed to generate rapamycin sensitivity under aerobic condition. Upon submergence, yeast and human FKBP12 can significantly block cotyledon greening while Arabidopsis FKBP12 only retards plant growth in the presence of rapamycin, suggesting that hypoxia stress could partially restore the functions of AtFKBP12 to bridge the interaction between rapamycin and TOR. To further determine if communication between TOR and auxin signaling exists in plants, yeast FKBP12 was introduced into DR5::GUS homozygous plants. The transgenic plants DR5/BP12 were then treated with rapamycin or KU63794 (a new inhibitor of TOR). GUS staining showed that the auxin content of root tips decreased compared to the control. DR5/BP12 plants lost sensitivity to auxin after treatment with rapamycin. Auxin-defective phenotypes, including short primary roots, fewer lateral roots, and loss of gravitropism, occurred in DR5/BP12 plants when seedlings were treated with rapamycin+KU63794. This indicated that the combination of rapamycin and KU63794 can significantly inhibit TOR and auxin signaling in DR5/BP12 plants. These studies demonstrate that TOR is essential for auxin signaling transduction in Arabidopsis.
Keywords: FKBP12; KU63794; auxin; rapamycin; target of rapamycin.
Figures






References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

