Preferred transduction with AAV8 and AAV9 via thalamic administration in the MPS IIIB model: A comparison of four rAAV serotypes
- PMID: 27014573
- PMCID: PMC4789330
- DOI: 10.1016/j.ymgmr.2015.11.006
Preferred transduction with AAV8 and AAV9 via thalamic administration in the MPS IIIB model: A comparison of four rAAV serotypes
Abstract
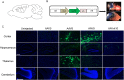
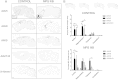
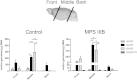
Sanfilippo syndrome type B (MPS IIIB) is a lysosomal storage disease caused by a deficiency of N-acetyl-glucosaminidase (NAGLU) activity. Since early therapeutic intervention is likely to yield the most efficacious results, we sought to determine the possible therapeutic utility of rAAV in early gene therapy based interventions. Currently, the application of recombinant adeno-associated virus (AAV) vectors is one of the most widely used gene transfer systems, and represents a promising approach in the treatment of MPS IIIB. From a translational standpoint, a minimally invasive, yet highly efficient method of vector administration is ideal. The thalamus is thought to be the switchboard for signal relay in the central nervous system (CNS) and therefore represents an attractive target. To identify an optimal AAV vector for early therapeutic intervention, and establish whether thalamic administration represents a feasible therapeutic approach, we performed a comprehensive assessment of transduction and biodistribution profiles of four green fluorescent protein (GFP) bearing rAAV serotypes, -5, -8, -9 and -rh10, administered bilaterally into the thalamus. Of the four serotypes compared, AAV8 and -9 proved superior to AAV5 and -rh10 both in biodistribution and transduction efficiency profiles. Genotype differences in transduction efficiency and biodistribution patterns were also observed. Importantly, we conclude that AAV8 and to a lesser extent, AAV9 represent preferable candidates for early gene therapy based intervention in the treatment of MPS IIIB. We also highlight the feasibility of thalamic rAAV administration, and conclude that this method results in moderate rAAV biodistribution with limited treatment capacity, thus suggesting a need for alternate methods of vector delivery.
Keywords: AAV; ANOVA, analysis of variance; Adeno-associated virus; BBB, blood brain barrier; CNS, central nervous system; GFAP, glial fibrillary acidic protein; Gene therapy; Lysosomal storage disease; MPS IIIB; MPS IIIB, mucopolysaccharidosis type III B; Mucopolysaccharidosis type III B; NeuN, neuronal nuclear protein; hGFP, humanized green fluorescent protein; rAAV, recombinant adeno-associated virus.
Figures

References
-
- Yogalingam G., Hopwood J.J. Molecular genetics of mucopolysaccharidosis type IIIA and IIIB: diagnostic, clinical, and biological implications. Hum. Mutat. 2001;18:264–281. - PubMed
-
- Gilkes J.A., Heldermon C.D. Mucopolysaccharidosis III (Sanfilippo syndrome) — disease presentation and experimental therapies. Pediatr. Endocrinol. Rev. 2014;12(Suppl. 1):133–140. - PubMed
-
- Flotte T.R. Gene therapy progress and prospects: recombinant adeno-associated virus (rAAV) vectors. Gene Ther. 2004;11:805–810. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

