Glycomic Approaches for the Discovery of Targets in Gastrointestinal Cancer
- PMID: 27014630
- PMCID: PMC4783390
- DOI: 10.3389/fonc.2016.00055
Glycomic Approaches for the Discovery of Targets in Gastrointestinal Cancer
Abstract
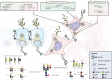
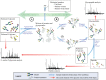
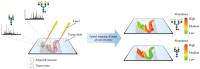
Gastrointestinal (GI) cancer is the most common group of malignancies and many of its types are among the most deadly. Various glycoconjugates have been used in clinical practice as serum biomarker for several GI tumors, however, with limited diagnose application. Despite the good accessibility by endoscopy of many GI organs, the lack of reliable serum biomarkers often leads to late diagnosis of malignancy and consequently low 5-year survival rates. Recent advances in analytical techniques have provided novel glycoproteomic and glycomic data and generated functional information and putative biomarker targets in oncology. Glycosylation alterations have been demonstrated in a series of glycoconjugates (glycoproteins, proteoglycans, and glycosphingolipids) that are involved in cancer cell adhesion, signaling, invasion, and metastasis formation. In this review, we present an overview on the major glycosylation alterations in GI cancer and the current serological biomarkers used in the clinical oncology setting. We further describe recent glycomic studies in GI cancer, namely gastric, colorectal, and pancreatic cancer. Moreover, we discuss the role of glycosylation as a modulator of the function of several key players in cancer cell biology. Finally, we address several state-of-the-art techniques currently applied in this field, such as glycomic and glycoproteomic analyses, the application of glycoengineered cell line models, microarray and proximity ligation assay, and imaging mass spectrometry, and provide an outlook to future perspectives and clinical applications.
Keywords: colorectal cancer; gastric cancer; glycan biomarkers; glycomics; imaging mass spectrometry; microarray; pancreatic cancer; proximity ligation assay.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

