At Least Three Transporters Likely Mediate Threonine Uptake Needed for Mouse Embryonic Stem Cell Proliferation
- PMID: 27014692
- PMCID: PMC4791362
- DOI: 10.3389/fcell.2016.00017
At Least Three Transporters Likely Mediate Threonine Uptake Needed for Mouse Embryonic Stem Cell Proliferation
Abstract
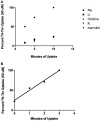
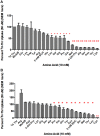
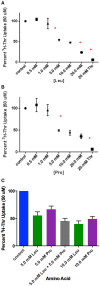
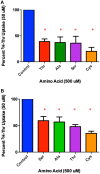
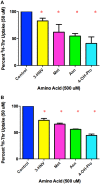
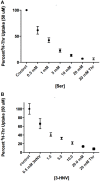
Stem cells are at the forefront of current regenerative and biomedical research. Thus, there exists an imperative and urgent need to understand the mechanisms that drive stem cell function in order to exploit their use as a therapeutic tool. Amino acids are potent inducers of signaling cascades that drive stem cell proliferation and differentiation. With a focus on mouse embryonic stem (mES) cells, Threonine (Thr) is the only amino acid required in culture media for mES cell proliferation. Current research associates this need for Thr with threonine dehydrogenase (TDH), which catabolizes Thr to glycine and acetyl-CoA in mES cells. This theory depends, in part, on the ability of 3- hydroxynorvaline (3-HNV) to inhibit both TDH and mES cell proliferation. However, the concentration of 3-HNV needed to inhibit mES cell proliferation is more than an order of magnitude less than its apparent Ki for TDH inhibition. Additionally, 3-HNV inhibits human embryonic stem (hES) cell proliferation, but hES cells do not express a functional tdh gene. Such findings indicate another mechanism for Thr stimulated mES and hES cell proliferation. Since amino acid transporters may be inducers of signaling cascades, we characterized the Thr transport systems in mES cells. We found that there is a Na(+)-dependent and a Na(+)-independent component of substrate-saturable transport, with the Na(+)-dependent component predominating. We also found that of 20 amino acids tested, the amino acids that were the strongest inhibitors of the Na(+)-dependent component of radiolabeled Thr transport were Ser, Cys, 4-OH-Pro, Asn, Met, and non-radiolabeled Thr itself. Such findings are consistent with characteristics of the ASC transport system, suggesting that this ASC system is responsible for the majority of Thr transport in mES cells. We confirmed expression of mRNA encoding the ASC system transporters, ASCT1 and ASCT2, in mES cells using RT-PCR. In conclusion, mES cells likely express at least three transporters of Thr; at least two Na(+)-dependent transporters and one Na(+)-independent one.
Keywords: amino acid transport system ASC; amino acid transport system L; cell proliferation; mES cells; threonine.
Figures

References
-
- Bröer A., Brookes N., Ganapathy V., Dimmer K. S., Wagner C. A., Lang F., et al. . (1999). The astroglial ASCT2 amino acid transporter as a mediator of glutamine efflux. J. Neurochem. 73, 2184–2194. - PubMed
-
- Darling P. B., Grunow J., Rafii M., Brookes S., Ball R. O., Pencharz P. B. (2000). Threonine dehydrogenase is a minor degradative pathway of threonine catabolism in adult humans. Am. J. Physiol. Endocrinol. Metab. 278, E877–E884. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

