Hepatitis C Disease Burden in the United States in the era of oral direct-acting antivirals
- PMID: 27015107
- PMCID: PMC5035714
- DOI: 10.1002/hep.28571
Hepatitis C Disease Burden in the United States in the era of oral direct-acting antivirals
Abstract
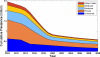
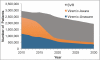
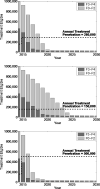
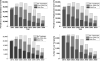
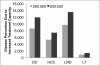
Oral direct-acting antivirals (DAAs) represent a major advance in hepatitis C virus (HCV) treatment. Along with recent updates in HCV screening policy and expansions in insurance coverage, treatment demand in the United States is changing rapidly. Our objective was to project the characteristics and number of people needing antiviral treatment and HCV-associated disease burden in the era of oral DAAs. We used a previously developed and validated Hepatitis C Disease Burden Simulation model (HEP-SIM). HEP-SIM simulated the actual clinical management of HCV from 2001 onward, which included antiviral treatment with pegylated interferon (Peg-IFN)-based therapies as well as the recent oral DAAs, risk-based and birth-cohort HCV screening, and the impact of the Affordable Care Act. We also simulated two hypothetical scenarios-no treatment and treatment with Peg-IFN-based therapies only. We estimated that in 2010, 2.5 (95% confidence interval [CI], 1.9-3.1) million noninstitutionalized people were viremic, which dropped to 1.9 (95% CI, 1.4-2.6) million in 2015, and projected to drop below 1 million by 2020. A total of 1.8 million HCV patients will receive HCV treatment from the launch of oral DAAs in 2014 until 2030. Based on current HCV management practices, it will take 4-6 years to treat the majority of patients aware of their disease. However, 560,000 patients would still remain unaware by 2020. Even in the oral DAA era, 320,000 patients will die, 157,000 will develop hepatocellular carcinoma, and 203,000 will develop decompensated cirrhosis in the next 35 years.
Conclusions: HCV-associated disease burden will still remain substantial in the era of oral DAAs. Increasing HCV screening and treatment capacity is essential to further decreasing HCV burden in the United States. (Hepatology 2016;64:1442-1450).
© 2016 by the American Association for the Study of Liver Diseases.
Figures
Comment in
-
The end of the hepatitis C burden: Really?Hepatology. 2016 Nov;64(5):1404-1407. doi: 10.1002/hep.28758. Epub 2016 Aug 25. Hepatology. 2016. PMID: 27486957 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

