The Incidence, Classification, and Management of Acute Adverse Reactions to the Low-Osmolar Iodinated Contrast Media Isovue and Ultravist in Contrast-Enhanced Computed Tomography Scanning
- PMID: 27015204
- PMCID: PMC4998399
- DOI: 10.1097/MD.0000000000003170
The Incidence, Classification, and Management of Acute Adverse Reactions to the Low-Osmolar Iodinated Contrast Media Isovue and Ultravist in Contrast-Enhanced Computed Tomography Scanning
Abstract
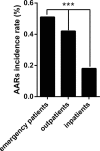
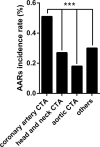
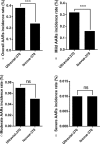
Some epidemiologic surveillance studies have recorded adverse drug reactions to radiocontrast agents. We aimed to investigate the incidence and management of acute adverse reactions (AARs) to Ultravist-370 and Isovue-370 in patients who underwent contrast-enhanced computed tomography (CT) scanning.Data from 137,473 patients were analyzed. They had undergone enhanced CT scanning with intravenous injection of Ultravist-370 or Isovue-370 during the period of January 1, 2006 to December 31, 2012 in our hospital. We investigated and classified AARs according to the American College of Radiology and the Chinese Society of Radiology (CSR) guidelines for iodinated contrast media. We analyzed risk factors for AARs and compared the AARs induced by Ultravist-370 and Isovue-370.Four hundred and twenty-eight (0.31%) patients experienced AARs, which included 330 (0.24%) patients with mild AARs, 82 (0.06%) patients with moderate AARs, and 16 (0.01%) patients with severe AARs (including 3 cases of cardiac arrest and one case of death). The incidence of AARs was higher with Ultravist-370 than with Isovue-370 (0.38% vs 0.24%, P < 0.001), but only for mild AARs (0.32% vs 0.16%, P < 0.001). Analyses on risk factors indicated that female patients (n = 221, 0.43%, P < 0.001), emergency patients (n = 11, 0.51%, P < 0.001), elderly patients aged 50 to 60 years (n = 135, 0.43%, P < 0.001), and patients who underwent coronary computed tomography angiography (CTA) (n = 55, 0.51%, P < 0.001) had a higher risk of AARs. Cutaneous manifestations (50.52%)-especially rash (59.74%)-were the most frequent mild AARs. Cardiovascular manifestations accounted for most moderate and severe AARs (62.91% and 48.28%, respectively). After proper management, the symptoms and signs of 96.5% of the AARs resolved within 24 hours without sequelae.Ultravist-370 and Isovue-370 are safe for patients undergoing enhanced CT scanning. The incidence of AARs is higher with Ultravist-370 than with Isovue-370, but this difference is limited only to the mild AARs. The incidence of AARs could be affected by multiple factors.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
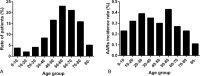
References
-
- Berner J, Poletti PA, Becker CD, et al. Adverse reactions to iodinated contrast media: how to prevent them? Rev Med Suisse 2009; 5:2016–2018.2020-2021. - PubMed
-
- ESUR Guidelines on contrast media. Version 9.0, 2014. www.esur.org Accessed September 15, 2015.
-
- Palkowitsch PK, Bostelmann S, Lengsfeld P. Safety and tolerability of iopromide intravascular use: a pooled analysis of three non-interventional studies in 132,012 patients. Acta Radiologica 2014; 55:707–714. - PubMed
-
- Haussler MD. Safety and patient comfort with iodixanol: a postmarketing surveillance study in 9515 patients undergoing diagnostic CT examinations. Acta Radiol 2010; 51:924–933. - PubMed
-
- Palkowitsch P, Lengsfeld P, Stauch K, et al. Safety and diagnostic image quality of iopromide: results of a large non-interventional observational study of European and Asian patients (IMAGE). Acta Radiol 2012; 53:179–186. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

