Dried Blood Spot Methodology in Combination With Liquid Chromatography/Tandem Mass Spectrometry Facilitates the Monitoring of Teriflunomide
- PMID: 27015245
- PMCID: PMC4949010
- DOI: 10.1097/FTD.0000000000000302
Dried Blood Spot Methodology in Combination With Liquid Chromatography/Tandem Mass Spectrometry Facilitates the Monitoring of Teriflunomide
Abstract
Background: Teriflunomide, a once-daily oral immunomodulator approved for treatment of relapsing-remitting multiple sclerosis, is eliminated slowly from plasma. If necessary to rapidly lower plasma concentrations of teriflunomide, an accelerated elimination procedure using cholestyramine or activated charcoal may be used. The current bioanalytical assay for determination of plasma teriflunomide concentration requires laboratory facilities for blood centrifugation and plasma storage. An alternative method, with potential for greater convenience, is dried blood spot (DBS) methodology. Analytical and clinical validations are required to switch from plasma to DBS (finger-prick sampling) methodology.
Methods: Using blood samples from healthy subjects, an LC-MS/MS assay method for quantification of teriflunomide in DBS over a range of 0.01-10 mcg/mL was developed and validated for specificity, selectivity, accuracy, precision, reproducibility, and stability. Results were compared with those from the current plasma assay for determination of plasma teriflunomide concentration.
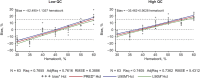
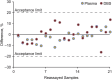
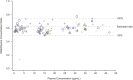
Results: Method was specific and selective relative to endogenous compounds, with process efficiency ∼88%, and no matrix effect. Inaccuracy and imprecision for intraday and interday analyses were <15% at all concentrations tested. Quantification of teriflunomide in DBS assay was not affected by blood deposit volume and punch position within spot, and hematocrit level had a limited but acceptable effect on measurement accuracy. Teriflunomide was stable for at least 4 months at room temperature, and for at least 24 hours at 37°C with and without 95% relative humidity, to cover sampling, drying, and shipment conditions in the field. The correlation between DBS and plasma concentrations (R = 0.97), with an average blood to plasma ratio of 0.59, was concentration independent and constant over time.
Conclusions: DBS sampling is a simple and practical method for monitoring teriflunomide concentrations.
Conflict of interest statement
A. Filali-Ansary, C. Lunven, Y.-J. Beyer, A. Delfolie, and G.-J. Sanderink are employees of Sanofi R&D, France. S. Turpault and A. O'Brien are employees of Sanofi R&D. N. Boyanova is an employee of Sanofi Genzyme. F. Baldinetti is a former employee of Sanofi Genzyme.
Figures


References
-
- Aubagio [teriflunomide film-coated tablets]. EU Summary of Product Characteristics. Sanofi-Aventis; 2015. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Info.... Accessed August 21, 2015.
-
- Guthrie R, Susi A. A simple phenylalanine method for detecting phenylketonuria in large populations of new born infants. Pediatrics. 1963;32:338–343. - PubMed
-
- Sharma A, Jaiswal S, Shukla M, et al. Dried blood spots: concepts, present status, and future perspectives in bioanalysis. Drug Test Anal. 2014;6:399–414. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

