Hemotin, a Regulator of Phagocytosis Encoded by a Small ORF and Conserved across Metazoans
- PMID: 27015288
- PMCID: PMC4807881
- DOI: 10.1371/journal.pbio.1002395
Hemotin, a Regulator of Phagocytosis Encoded by a Small ORF and Conserved across Metazoans
Abstract
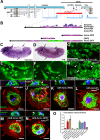
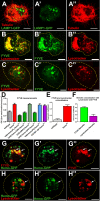
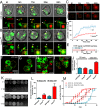
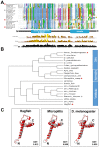
Translation of hundreds of small ORFs (smORFs) of less than 100 amino acids has recently been revealed in vertebrates and Drosophila. Some of these peptides have essential and conserved cellular functions. In Drosophila, we have predicted a particular smORF class encoding ~80 aa hydrophobic peptides, which may function in membranes and cell organelles. Here, we characterise hemotin, a gene encoding an 88aa transmembrane smORF peptide localised to early endosomes in Drosophila macrophages. hemotin regulates endosomal maturation during phagocytosis by repressing the cooperation of 14-3-3ζ with specific phosphatidylinositol (PI) enzymes. hemotin mutants accumulate undigested phagocytic material inside enlarged endo-lysosomes and as a result, hemotin mutants have reduced ability to fight bacteria, and hence, have severely reduced life span and resistance to infections. We identify Stannin, a peptide involved in organometallic toxicity, as the Hemotin functional homologue in vertebrates, showing that this novel regulator of phagocytic processing is widely conserved, emphasizing the significance of smORF peptides in cell biology and disease.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
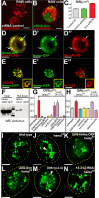
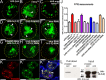
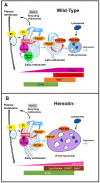
References
-
- Aderem A, Underhill DM (1999) Mechanisms of phagocytosis in macrophages. Annu Rev Immunol 17: 593–623. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

