Metastatic Latency and Immune Evasion through Autocrine Inhibition of WNT
- PMID: 27015306
- PMCID: PMC4808520
- DOI: 10.1016/j.cell.2016.02.025
Metastatic Latency and Immune Evasion through Autocrine Inhibition of WNT
Abstract
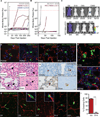
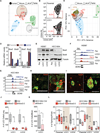
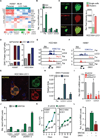
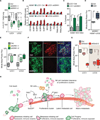
Metastasis frequently develops years after the removal of a primary tumor, from a minority of disseminated cancer cells that survived as latent entities through unknown mechanisms. We isolated latency competent cancer (LCC) cells from early stage human lung and breast carcinoma cell lines and defined the mechanisms that suppress outgrowth, support long-term survival, and maintain tumor-initiating potential in these cells during the latent metastasis stage. LCC cells show stem-cell-like characteristics and express SOX2 and SOX9 transcription factors, which are essential for their survival in host organs under immune surveillance and for metastatic outgrowth under permissive conditions. Through expression of the WNT inhibitor DKK1, LCC cells self-impose a slow-cycling state with broad downregulation of ULBP ligands for NK cells and evasion of NK-cell-mediated clearance. By expressing a Sox-dependent stem-like state and actively silencing WNT signaling, LCC cells can enter quiescence and evade innate immunity to remain latent for extended periods.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures



Comment in
-
Autocrine WNT Inhibition Drives Immune Evasion and Latency of Metastases.Cancer Discov. 2016 May;6(5):471. doi: 10.1158/2159-8290.CD-RW2016-061. Epub 2016 Apr 7. Cancer Discov. 2016. PMID: 27056833
References
-
- Aslakson CJ, Miller FR. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 1992;52:1399–1405. - PubMed
-
- Bradley M, Zeytun A, Rafi-Janajreh A, Nagarkatti PS, Nagarkatti M. Role of spontaneous and interleukin-2-induced natural killer cell activity in the cytotoxicity and rejection of Fas+ and Fas− tumor cells. Blood. 1998;92:4248–4255. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

