The retinoblastoma protein regulates hypoxia-inducible genetic programs, tumor cell invasiveness and neuroendocrine differentiation in prostate cancer cells
- PMID: 27015368
- PMCID: PMC5029701
- DOI: 10.18632/oncotarget.8301
The retinoblastoma protein regulates hypoxia-inducible genetic programs, tumor cell invasiveness and neuroendocrine differentiation in prostate cancer cells
Abstract
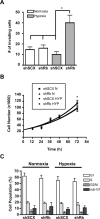
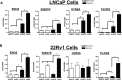
Loss of tumor suppressor proteins, such as the retinoblastoma protein (Rb), results in tumor progression and metastasis. Metastasis is facilitated by low oxygen availability within the tumor that is detected by hypoxia inducible factors (HIFs). The HIF1 complex, HIF1α and dimerization partner the aryl hydrocarbon receptor nuclear translocator (ARNT), is the master regulator of the hypoxic response. Previously, we demonstrated that Rb represses the transcriptional response to hypoxia by virtue of its association with HIF1. In this report, we further characterized the role Rb plays in mediating hypoxia-regulated genetic programs by stably ablating Rb expression with retrovirally-introduced short hairpin RNA in LNCaP and 22Rv1 human prostate cancer cells. DNA microarray analysis revealed that loss of Rb in conjunction with hypoxia leads to aberrant expression of hypoxia-regulated genetic programs that increase cell invasion and promote neuroendocrine differentiation. For the first time, we have established a direct link between hypoxic tumor environments, Rb inactivation and progression to late stage metastatic neuroendocrine prostate cancer. Understanding the molecular pathways responsible for progression of benign prostate tumors to metastasized and lethal forms will aid in the development of more effective prostate cancer therapies.
Keywords: hypoxia; invasion; neuroendocrine differentiation; prostate cancer; retinoblastoma protein.
Conflict of interest statement
The authors have no conflicts of interest to report.
Figures





Similar articles
-
A TRIP230-retinoblastoma protein complex regulates hypoxia-inducible factor-1α-mediated transcription and cancer cell invasion.PLoS One. 2014 Jun 11;9(6):e99214. doi: 10.1371/journal.pone.0099214. eCollection 2014. PLoS One. 2014. PMID: 24919196 Free PMC article.
-
Annexin-A7 protects normal prostate cells and induces distinct patterns of RB-associated cytotoxicity in androgen-sensitive and -resistant prostate cancer cells.Int J Cancer. 2009 Dec 1;125(11):2528-39. doi: 10.1002/ijc.24592. Int J Cancer. 2009. PMID: 19610065
-
REST reduction is essential for hypoxia-induced neuroendocrine differentiation of prostate cancer cells by activating autophagy signaling.Oncotarget. 2016 May 3;7(18):26137-51. doi: 10.18632/oncotarget.8433. Oncotarget. 2016. PMID: 27034167 Free PMC article.
-
The role of tumor suppressor dysregulation in prostate cancer progression.Curr Drug Targets. 2013 Apr;14(4):460-71. doi: 10.2174/1389450111314040007. Curr Drug Targets. 2013. PMID: 23410128 Review.
-
Hypoxia-inducible factor-1 in human breast and prostate cancer.Endocr Relat Cancer. 2006 Sep;13(3):739-49. doi: 10.1677/erc.1.00728. Endocr Relat Cancer. 2006. PMID: 16954428 Review.
Cited by
-
Molecular profiling stratifies diverse phenotypes of treatment-refractory metastatic castration-resistant prostate cancer.J Clin Invest. 2019 Jul 30;129(10):4492-4505. doi: 10.1172/JCI128212. J Clin Invest. 2019. PMID: 31361600 Free PMC article.
-
Molecular Links Between Angiogenesis and Neuroendocrine Phenotypes in Prostate Cancer Progression.Front Oncol. 2020 Jan 21;9:1491. doi: 10.3389/fonc.2019.01491. eCollection 2019. Front Oncol. 2020. PMID: 32039001 Free PMC article. Review.
-
Antagonists of the serotonin receptor 5A target human breast tumor initiating cells.BMC Cancer. 2020 Aug 5;20(1):724. doi: 10.1186/s12885-020-07193-6. BMC Cancer. 2020. PMID: 32758183 Free PMC article.
-
The number of titrated microRNA species dictates ceRNA regulation.Nucleic Acids Res. 2018 May 18;46(9):4354-4369. doi: 10.1093/nar/gky286. Nucleic Acids Res. 2018. PMID: 29684207 Free PMC article.
-
Reduction of two histone marks, H3k9me3 and H3k27me3 by epidrug induces neuroendocrine differentiation in prostate cancer.J Cell Biochem. 2018 Apr;119(4):3697-3705. doi: 10.1002/jcb.26586. Epub 2018 Jan 9. J Cell Biochem. 2018. PMID: 29236331 Free PMC article.
References
-
- Vaupel P, Kelleher DK, Hockel M. Oxygen status of malignant tumors: pathogenesis of hypoxia and significance for tumor therapy. Semin Oncol. 2001;28:29–35. - PubMed
-
- Labrecque MP, Prefontaine GG, Beischlag TV. The aryl hydrocarbon receptor nuclear translocator (ARNT) family of proteins: transcriptional modifiers with multi-functional protein interfaces. Curr Mol Med. 2013;13:1047–1065. - PubMed
-
- Beischlag TV, Taylor RT, Rose DW, Yoon D, Chen Y, Lee WH, Rosenfeld MG, Hankinson O. Recruitment of thyroid hormone receptor/retinoblastoma-interacting protein 230 by the aryl hydrocarbon receptor nuclear translocator is required for the transcriptional response to both dioxin and hypoxia. J Biol Chem. 2004;279:54620–54628. - PubMed
-
- Wang F, Zhang R, Beischlag TV, Muchardt C, Yaniv M, Hankinson O. Roles of Brahma and Brahma/SWI2-related gene 1 in hypoxic induction of the erythropoietin gene. J Biol Chem. 2004;279:46733–46741. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

