The frequency of multipotent CD133(+)CD45RA(-)CD34(+) hematopoietic stem cells is not increased in fetal liver compared with adult stem cell sources
- PMID: 27016273
- PMCID: PMC5565165
- DOI: 10.1016/j.exphem.2016.02.011
The frequency of multipotent CD133(+)CD45RA(-)CD34(+) hematopoietic stem cells is not increased in fetal liver compared with adult stem cell sources
Abstract
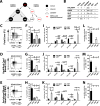
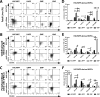
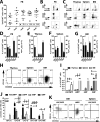
The cell surface marker CD133 has been used to describe a revised model of adult human hematopoiesis, with hematopoietic stem cells and multipotent progenitors (HSCs/MPPs: CD133(+)CD45RA(-)CD34(+)) giving rise to lymphomyeloid-primed progenitors (LMPPs: CD133(+)CD45RA(+)CD34(+)) and erythromyeloid progenitors (EMPs: CD133(low)CD45RA(-)CD34(+)). Because adult and fetal hematopoietic stem and progenitor cells (HSPCs) differ in their gene expression profile, differentiation capabilities, and cell surface marker expression, we were interested in whether the reported segregation of lineage potentials in adult human hematopoiesis would also apply to human fetal liver. CD133 expression was easily detected in human fetal liver cells, and the defined hematopoietic subpopulations were similar to those found for adult HSPCs. Fetal HSPCs were enriched for EMPs and HSCs/MPPs, which were primed toward erythromyeloid differentiation. However, the frequency of multipotent CD133(+)CD45RA(-)CD34(+) HSPCs was much lower than previously reported and comparable to that of umbilical cord blood. We noted that engraftment in NSG (NOD scid gamma [NOD.Cg-Prkdc(scid) Il2rg(tm1Wjl)/SzJ]) mice was driven mostly by LMPPs, confirming recent findings that repopulation in mice is not a unique feature of multipotent HSCs/MPPs. Thus, our data challenge the general assumption that human fetal liver contains a greater percentage of multipotent HSCs/MPPs than any adult HSC source, and the mouse model may have to be re-evaluated with respect to the type of readout it provides.
Copyright © 2016. Published by Elsevier Inc.
Conflict of interest statement
The authors declare that there are no conflicts of interest
Figures
Similar articles
-
Human fetal hepatic progenitor cells are distinct from, but closely related to, hematopoietic stem/progenitor cells.Stem Cells. 2013 Jun;31(6):1160-9. doi: 10.1002/stem.1359. Stem Cells. 2013. PMID: 23404852
-
CD133 allows elaborated discrimination and quantification of haematopoietic progenitor subsets in human haematopoietic stem cell transplants.Br J Haematol. 2015 Jun;169(6):868-78. doi: 10.1111/bjh.13362. Epub 2015 Mar 29. Br J Haematol. 2015. PMID: 25819405
-
Human mesenchymal and murine stromal cells support human lympho-myeloid progenitor expansion but not maintenance of multipotent haematopoietic stem and progenitor cells.Cell Cycle. 2016;15(4):540-5. doi: 10.1080/15384101.2015.1128591. Cell Cycle. 2016. PMID: 26818432 Free PMC article.
-
[Updated human hematopoietic stem cell findings: purification of human hematopoietic stem cells and elucidation of their hierarchy].Rinsho Ketsueki. 2018;59(10):1861-1871. doi: 10.11406/rinketsu.59.1861. Rinsho Ketsueki. 2018. PMID: 30305486 Review. Japanese.
-
Biological and molecular evidence for existence of lymphoid-primed multipotent progenitors.Ann N Y Acad Sci. 2007 Jun;1106:89-94. doi: 10.1196/annals.1392.023. Epub 2007 Apr 18. Ann N Y Acad Sci. 2007. PMID: 17442777 Review.
Cited by
-
Mouse models in hematopoietic stem cell gene therapy and genome editing.Biochem Pharmacol. 2020 Apr;174:113692. doi: 10.1016/j.bcp.2019.113692. Epub 2019 Nov 6. Biochem Pharmacol. 2020. PMID: 31705854 Free PMC article. Review.
-
Purification of Human CD34+CD90+ HSCs Reduces Target Cell Population and Improves Lentiviral Transduction for Gene Therapy.Mol Ther Methods Clin Dev. 2020 Jul 15;18:679-691. doi: 10.1016/j.omtm.2020.07.010. eCollection 2020 Sep 11. Mol Ther Methods Clin Dev. 2020. PMID: 32802914 Free PMC article.
-
Human fetal liver cultures support multiple cell lineages that can engraft immunodeficient mice.Open Biol. 2017 Dec;7(12):170108. doi: 10.1098/rsob.170108. Open Biol. 2017. PMID: 29237808 Free PMC article.
-
Flow Cytometric Identification of Hematopoietic and Leukemic Blast Cells for Tailored Clinical Follow-Up of Acute Myeloid Leukemia.Int J Mol Sci. 2022 Sep 11;23(18):10529. doi: 10.3390/ijms231810529. Int J Mol Sci. 2022. PMID: 36142442 Free PMC article. Review.
-
Suppression of luteinizing hormone enhances HSC recovery after hematopoietic injury.Nat Med. 2018 Feb;24(2):239-246. doi: 10.1038/nm.4470. Epub 2018 Jan 8. Nat Med. 2018. PMID: 29309056 Free PMC article.
References
-
- Görgens A, Radtke S, Möllmann M, et al. Revision of the human hematopoietic tree: granulocyte subtypes derive from distinct hematopoietic lineages. Cell reports. 2013;3:1539–1552. - PubMed
-
- Radtke S, Görgens A, Kordelas L, et al. CD133 allows elaborated discrimination and quantification of haematopoietic progenitor subsets in human haematopoietic stem cell transplants. British journal of haematology. 2015;169:868–878. - PubMed
-
- Rollini P, Faes-Van’t Hull E, Kaiser S, Kapp U, Leyvraz S. Phenotypic and functional analysis of human fetal liver hematopoietic stem cells in culture. Stem Cells Dev. 2007;16:281–296. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

