NINA-LAMP compared to microscopy, RDT, and nested PCR for the detection of imported malaria
- PMID: 27017271
- PMCID: PMC4862928
- DOI: 10.1016/j.diagmicrobio.2015.11.009
NINA-LAMP compared to microscopy, RDT, and nested PCR for the detection of imported malaria
Abstract
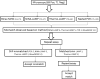
Microscopy and field adaptable rapid diagnostic tests (RDTs) are not sensitive and specific in certain conditions such as poor training of microscopists, lack of electricity, or lower sensitivity in the detection of non-falciparum malaria. More sensitive point-of-care testing (POCT) would reduce delays in diagnosis and initiation of therapy. In the current study, we have evaluated the efficacy of noninstrumented nucleic acid amplification (NINA) coupled with loop-mediated isothermal amplification (LAMP) for detection of traveler's malaria (n=140) in comparison with microscopy, nested PCR, and the only Food and Drug Administration-approved rapid diagnostic test. NINA-LAMP was 100% sensitive and 98.6% specific when compared to nested PCR. For non-falciparum detection, NINA-LAMP sensitivity was 100% sensitive compared to nested PCR, whereas RDT sensitivity was 71%. LAMP is highly sensitive and specific for symptomatic malaria diagnosis regardless of species.
Keywords: LAMP; Malaria; Point of care test.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures

References
-
- Abdul-Ghani R, Al-Mekhlafi AM, Karanis P. Loop-mediated isothermal amplification (LAMP) for malarial parasites of humans: would it come to clinical reality as a point-of-care test? Acta Trop. 2012;122:233–40. - PubMed
-
- Bacaner N, Stauffer B, Boulware DR, Walker PF, Keystone JS. Travel medicine considerations for North American immigrants visiting friends and relatives. JAMA. 2004;291:2856–64. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

