Diverse Regulation of the CreA Carbon Catabolite Repressor in Aspergillus nidulans
- PMID: 27017621
- PMCID: PMC4858783
- DOI: 10.1534/genetics.116.187872
Diverse Regulation of the CreA Carbon Catabolite Repressor in Aspergillus nidulans
Abstract
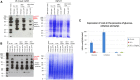
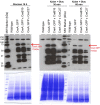
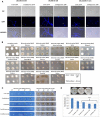
Carbon catabolite repression (CCR) is a process that selects the energetically most favorable carbon source in an environment. CCR represses the use of less favorable carbon sources when a better source is available. Glucose is the preferential carbon source for most microorganisms because it is rapidly metabolized, generating quick energy for growth. In the filamentous fungus Aspergillus nidulans, CCR is mediated by the transcription factor CreA, a C2H2 finger domain DNA-binding protein. The aim of this work was to investigate the regulation of CreA and characterize its functionally distinct protein domains. CreA depends in part on de novo protein synthesis and is regulated in part by ubiquitination. CreC, the scaffold protein in the CreB-CreC deubiquitination (DUB) complex, is essential for CreA function and stability. Deletion of select protein domains in CreA resulted in persistent nuclear localization and target gene repression. A region in CreA conserved between Aspergillus spp. and Trichoderma reesei was identified as essential for growth on various carbon, nitrogen, and lipid sources. In addition, a role of CreA in amino acid transport and nitrogen assimilation was observed. Taken together, these results indicate previously unidentified functions of this important transcription factor. These novel functions serve as a basis for additional research in fungal carbon metabolism with the potential aim to improve fungal industrial applications.
Keywords: Aspergillus nidulans; carbon catabolite repression; cellulases; protein domains; ubiquitination.
Copyright © 2016 by the Genetics Society of America.
Figures


References
-
- Arst H. N., Tollervey D., Dowzwer C. E. A., Kelly J. M., 1990. An inversion truncating the creA gene of Aspergillus nidulans results in carbon catabolite repression. Mol. Microbiol. 4: 851–854. - PubMed
-
- Brown N. A., Ries L. N. A., Goldman G. H., 2014. How nutritional status signalling coordinates metabolism and lignocellulolytic enzyme secretion. Fungal Genet. Biol. 72: 48–63. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

