Progression and metastasis of lung cancer
- PMID: 27018053
- PMCID: PMC4821869
- DOI: 10.1007/s10555-016-9618-0
Progression and metastasis of lung cancer
Abstract
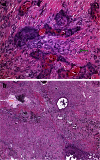
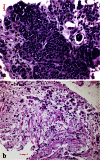
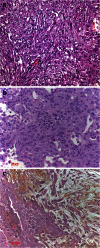
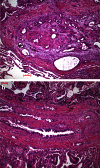
Metastasis in lung cancer is a multifaceted process. In this review, we will dissect the process in several isolated steps such as angiogenesis, hypoxia, circulation, and establishment of a metastatic focus. In reality, several of these processes overlap and occur even simultaneously, but such a presentation would be unreadable. Metastasis requires cell migration toward higher oxygen tension, which is based on changing the structure of the cell (epithelial-mesenchymal transition), orientation within the stroma and stroma interaction, and communication with the immune system to avoid attack. Once in the blood stream, cells have to survive trapping by the coagulation system, to survive shear stress in small blood vessels, and to find the right location for extravasation. Once outside in the metastatic locus, tumor cells have to learn the communication with the "foreign" stroma cells to establish vascular supply and again express molecules, which induce immune tolerance.
Keywords: Angiogenesis; Bone; Brain; Circulation; Epithelial-mesenchymal transition; Hypoxia; Lung cancer; Metastasis; Migration.
Figures







References
-
- Gabor S, Renner H, Popper H, Anegg U, Sankin O, Matzi V, Lindenmann J, Smolle Juttner FM. Invasion of blood vessels as significant prognostic factor in radically resected T1-3N0M0 non-small-cell lung cancer. European Journal of Cardio-Thoracic Surgery. 2004;25:439–442. doi: 10.1016/j.ejcts.2003.11.033. - DOI - PubMed
-
- Hendriks LE, Smit EF, Vosse BA, Mellema WW, Heideman DA, Bootsma GP, Westenend M, Pitz C, de Vries GJ, Houben R, Grunberg K, Bendek M, Speel EJ, Dingemans AM. EGFR mutated non-small cell lung cancer patients: more prone to development of bone and brain metastases? Lung Cancer. 2014;84:86–91. doi: 10.1016/j.lungcan.2014.01.006. - DOI - PubMed
-
- Wilbertz T, Wagner P, Petersen K, Stiedl AC, Scheble VJ, Maier S, Reischl M, Mikut R, Altorki NK, Moch H, Fend F, Staebler A, Bass AJ, Meyerson M, Rubin MA, Soltermann A, Lengerke C, Perner S. SOX2 gene amplification and protein overexpression are associated with better outcome in squamous cell lung cancer. Modern Pathology. 2011;24:944–953. doi: 10.1038/modpathol.2011.49. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

