Adrenergic activation attenuates astrocyte swelling induced by hypotonicity and neurotrauma
- PMID: 27018061
- PMCID: PMC4841273
- DOI: 10.1002/glia.22981
Adrenergic activation attenuates astrocyte swelling induced by hypotonicity and neurotrauma
Abstract
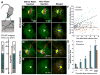
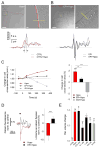
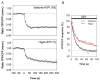
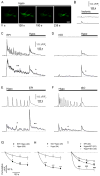
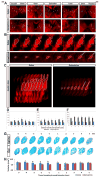
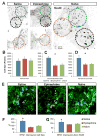
Edema in the central nervous system can rapidly result in life-threatening complications. Vasogenic edema is clinically manageable, but there is no established medical treatment for cytotoxic edema, which affects astrocytes and is a primary trigger of acute post-traumatic neuronal death. To test the hypothesis that adrenergic receptor agonists, including the stress stimulus epinephrine protects neural parenchyma from damage, we characterized its effects on hypotonicity-induced cellular edema in cortical astrocytes by in vivo and in vitro imaging. After epinephrine administration, hypotonicity-induced swelling of astrocytes was markedly reduced and cytosolic 3'-5'-cyclic adenosine monophosphate (cAMP) was increased, as shown by a fluorescence resonance energy transfer nanosensor. Although, the kinetics of epinephrine-induced cAMP signaling was slowed in primary cortical astrocytes exposed to hypotonicity, the swelling reduction by epinephrine was associated with an attenuated hypotonicity-induced cytosolic Ca(2+) excitability, which may be the key to prevent astrocyte swelling. Furthermore, in a rat model of spinal cord injury, epinephrine applied locally markedly reduced neural edema around the contusion epicenter. These findings reveal new targets for the treatment of cellular edema in the central nervous system.
Keywords: astrocytes; cerebral cortex; contusion trauma; cytotoxic edema; epinephrine; spinal cord.
© 2016 Wiley Periodicals, Inc.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
References
-
- Andrew RD, Labron MW, Boehnke SE, Carnduff L, Kirov SA. Physiological evidence that pyramidal neurons lack functional water channels. Cereb Cortex. 2007;17:787–802. - PubMed
-
- Azevedo FA, Carvalho LR, Grinberg LT, Farfel JM, Ferretti RE, Leite RE, Jacob Filho W, Lent R, Herculano-Houzel S. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol. 2009;513:532–541. - PubMed
-
- Bender AS, Neary JT, Norenberg MD. Involvement of second messengers and protein phosphorylation in astrocyte swelling. Can J Physiol Pharmacol. 1992;70(Suppl):S362–S366. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

