Effects of 1,25(OH)2 D3 and 25(OH)D3 on C2C12 Myoblast Proliferation, Differentiation, and Myotube Hypertrophy
- PMID: 27018098
- PMCID: PMC5111790
- DOI: 10.1002/jcp.25388
Effects of 1,25(OH)2 D3 and 25(OH)D3 on C2C12 Myoblast Proliferation, Differentiation, and Myotube Hypertrophy
Abstract
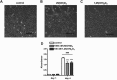
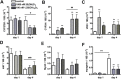
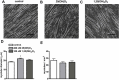
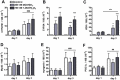
An adequate vitamin D status is essential to optimize muscle strength. However, whether vitamin D directly reduces muscle fiber atrophy or stimulates muscle fiber hypertrophy remains subject of debate. A mechanism that may affect the role of vitamin D in the regulation of muscle fiber size is the local conversion of 25(OH)D to 1,25(OH)2 D by 1α-hydroxylase. Therefore, we investigated in a murine C2C12 myoblast culture whether both 1,25(OH)2 D3 and 25(OH)D3 affect myoblast proliferation, differentiation, and myotube size and whether these cells are able to metabolize 25(OH)D3 and 1,25(OH)2 D3 . We show that myoblasts not only responded to 1,25(OH)2 D3 , but also to the precursor 25(OH)D3 by increasing their VDR mRNA expression and reducing their proliferation. In differentiating myoblasts and myotubes 1,25(OH)2 D3 as well as 25(OH)D3 stimulated VDR mRNA expression and in myotubes 1,25(OH)2 D3 also stimulated MHC mRNA expression. However, this occurred without notable effects on myotube size. Moreover, no effects on the Akt/mTOR signaling pathway as well as MyoD and myogenin mRNA levels were observed. Interestingly, both myoblasts and myotubes expressed CYP27B1 and CYP24 mRNA which are required for vitamin D3 metabolism. Although 1α-hydroxylase activity could not be shown in myotubes, after treatment with 1,25(OH)2 D3 or 25(OH)D3 myotubes showed strongly elevated CYP24 mRNA levels compared to untreated cells. Moreover, myotubes were able to convert 25(OH)D3 to 24R,25(OH)2 D3 which may play a role in myoblast proliferation and differentiation. These data suggest that skeletal muscle is not only a direct target for vitamin D3 metabolites, but is also able to metabolize 25(OH)D3 and 1,25(OH)2 D3 . J. Cell. Physiol. 231: 2517-2528, 2016. © 2016 The Authors. Journal of Cellular Physiology Published by Wiley Periodicals, Inc.
© 2016 The Authors. Journal of Cellular Physiology Published by Wiley Periodicals, Inc.
Figures

References
-
- Abboud M, Puglisi DA, Davies BN, Rybchyn M, Whitehead NP, Brock KE, Cole L, Gordon‐Thomson C, Fraser DR, Mason RS. 2013. Evidence for a specific uptake and retention mechanism for 25‐Hydroxyvitamin d (25OHD) in SkelMuscle cells. Endocrinology 154:3022–3030. - PubMed
-
- Anderson PH, Atkins GJ. 2008. The skeleton as an intracrine organ for vitamin D metabolism. Mol Aspects Med 29:397–406. - PubMed
-
- Atkins GJ, Anderson PH, Findlay DM, Welldon KJ, Vincent C, Zannettino AC, O'Loughlin PD, Morris HA. 2007. Metabolism of vitamin D3 in human osteoblasts: Evidence for autocrine and paracrine activities of 1α, 25‐dihydroxyvitamin D3 . Bone 40:1517–1528. - PubMed
-
- Bellido T, Drittanti L, Boland R, de Boland AR. 1987. The phospholipid and fatty acid composition of skeletal muscle cells during culture in the presence of vitamin D‐3 metabolites. Biochim Biophys Acta 922:162–169. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

