Biotechnological Chondroitin a Novel Glycosamminoglycan With Remarkable Biological Function on Human Primary Chondrocytes
- PMID: 27018169
- PMCID: PMC5084766
- DOI: 10.1002/jcb.25556
Biotechnological Chondroitin a Novel Glycosamminoglycan With Remarkable Biological Function on Human Primary Chondrocytes
Abstract
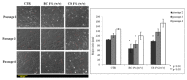
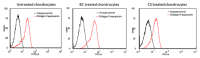
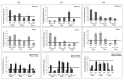
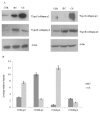
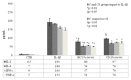
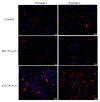
Cartilage tissue engineering, with in vitro expansion of autologus chondrocytes, is a promising technique for tissue regeneration and is a new potential strategy to prevent and/or treat cartilage damage (e.g., osteoarthritis). The aim of this study was (i) to investigate and compare the effects of new biotechnological chondroitin (BC) and a commercial extractive chondroitin sulfate (CS) on human chondrocytes in vitro culture; (ii) to evaluate the anti-inflammatory effects of the innovative BC compared to extractive CS. A chondrogenic cell population was isolated from human nasoseptal cartilage and in vitro cultures were studied through time-lapse video microscopy (TLVM), immunohistochemical staining and cytometry. In order to investigate the effect of BC and CS on phenotype maintainance, chondrogenic gene expression of aggrecan (AGN), of the transcriptor factor SOX9, of the types I and II collagen (COL1A1 and COL1A2), were quantified through transcriptional and protein evaluation at increasing cultivation time and passages. In addition to resemble the osteoarthritis-like in vitro model, chondrocytes were treated with IL-1β and the anti-inflammatory activity of BC and CS was assessed using cytokines quantification by multiplex array. BC significantly enhances cell proliferation also preserving chondrocyte phenotype increasing type II collagen expression up to 10 days of treatment and reduces inflammatory response in IL-1β treated chondrocytes respect to CS treated cells. Our results, taken together, suggest that this new BC is of foremost importance in translational medicine because it can be applied in novel scaffolds and pharmaceutical preparations aiming at cartilage pathology treatments such as the osteoarthritis. J. Cell. Biochem. 117: 2158-2169, 2016. © 2016 The Authors. Journal of Cellular Biochemistry Published by Wiley Periodicals, Inc.
Keywords: BIOTECHNOLOGICAL CHONDROITIN; CHONDROITIN SULFATE; ECM BIOMARKERS; INFLAMMATION IN VITRO MODEL; NASAL CHONDROCYTES; TIME-LAPSE VIDEOMICROSCOPY.
© 2016 The Authors. Journal of Cellular Biochemistry Published by Wiley Periodicals, Inc.
Figures


References
-
- Altman RD. 2010. Pharmacological therapies for osteoarthritis of the hand: A review of the evidence. Drugs Aging 27(9):729–745. - PubMed
-
- Bell DM, Leung KKH, Wheatly SC, Ng LJ, Zhou S. 1997. SOX9 directly regulates the type‐II collagen gene. Nat Genet 16:174–178. - PubMed
-
- Benya PD, Padilla SR, Nimni ME. 1978. Independent regulation of collagen types by chondrocytes during the loss of differentiated function in culture. Cell 15:1313–1321. - PubMed
-
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein‐dye binding. Anal Biochem 7(72):248–254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

