Pulmonary Targeting of Adeno-associated Viral Vectors by Next-generation Sequencing-guided Screening of Random Capsid Displayed Peptide Libraries
- PMID: 27018516
- PMCID: PMC4923327
- DOI: 10.1038/mt.2016.62
Pulmonary Targeting of Adeno-associated Viral Vectors by Next-generation Sequencing-guided Screening of Random Capsid Displayed Peptide Libraries
Abstract
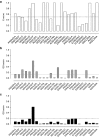
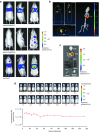
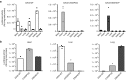
Vectors mediating strong, durable, and tissue-specific transgene expression are mandatory for safe and effective gene therapy. In settings requiring systemic vector administration, the availability of suited vectors is extremely limited. Here, we present a strategy to select vectors with true specificity for a target tissue from random peptide libraries displayed on adeno-associated virus (AAV) by screening the library under circulation conditions in a murine model. Guiding the in vivo screening by next-generation sequencing, we were able to monitor the selection kinetics and to determine the right time point to discontinue the screening process. The establishment of different rating scores enabled us to identify the most specifically enriched AAV capsid candidates. As proof of concept, a capsid variant was selected that specifically and very efficiently delivers genes to the endothelium of the pulmonary vasculature after intravenous administration. This technical approach of selecting target-specific vectors in vivo is applicable to any given tissue of interest and therefore has broad implications in translational research and medicine.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

