Recent Advances in the Synthesis of Cyclobutanes by Olefin [2 + 2] Photocycloaddition Reactions
- PMID: 27018601
- PMCID: PMC5025837
- DOI: 10.1021/acs.chemrev.5b00723
Recent Advances in the Synthesis of Cyclobutanes by Olefin [2 + 2] Photocycloaddition Reactions
Abstract
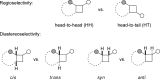
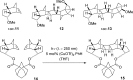
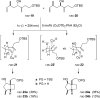
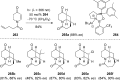
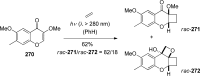
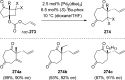
The [2 + 2] photocycloaddition is undisputedly the most important and most frequently used photochemical reaction. In this review, it is attempted to cover all recent aspects of [2 + 2] photocycloaddition chemistry with an emphasis on synthetically relevant, regio-, and stereoselective reactions. The review aims to comprehensively discuss relevant work, which was done in the field in the last 20 years (i.e., from 1995 to 2015). Organization of the data follows a subdivision according to mechanism and substrate classes. Cu(I) and PET (photoinduced electron transfer) catalysis are treated separately in sections 2 and 4 , whereas the vast majority of photocycloaddition reactions which occur by direct excitation or sensitization are divided within section 3 into individual subsections according to the photochemically excited olefin.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
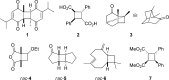

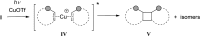
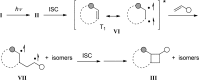

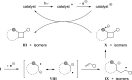
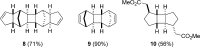

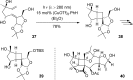
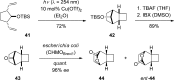
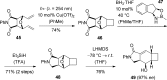

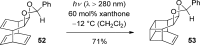

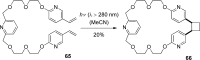

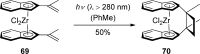
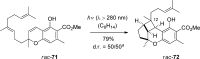

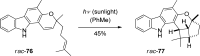




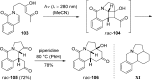
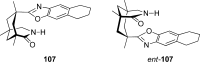
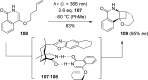
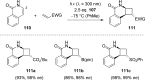

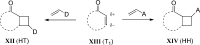
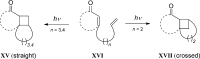
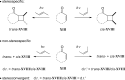
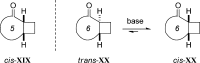






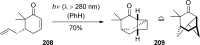
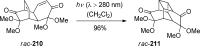

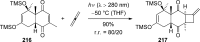
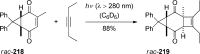

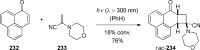
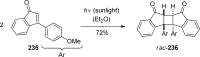

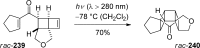
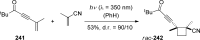



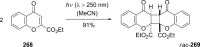





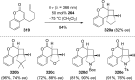
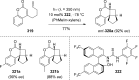
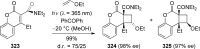

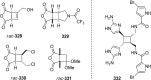







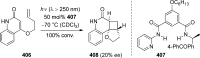
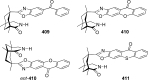
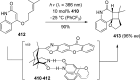

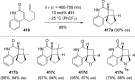

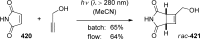
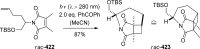
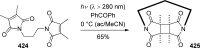


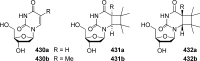
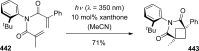


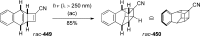

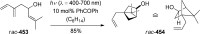
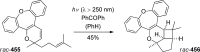


References
-
- Liebermann C. Ueber Polythymochinon. Ber. Dtsch. Chem. Ges. 1877, 10, 2177–2179. 10.1002/cber.187701002242. - DOI
-
- Rabinovich D.; Schmidt G. M. J. Topochemistry. Part XV. The Solid-State Photochemistry of p-Quinones. J. Chem. Soc. B 1967, 144–149. 10.1039/j29670000144. - DOI
-
- Liebermann C.; Ilinski M. Ueber Polythymochinon. Ber. Dtsch. Chem. Ges. 1885, 18, 3193–3201. 10.1002/cber.188501802272. - DOI
-
- Bertram J.; Kürsten R. Ueber das Vorkommen des Orthocumaraldehyd-methyläthers im Cassiaöl. J. Prakt. Chem. 1895, 51, 316–325. 10.1002/prac.18950510123. - DOI
-
- Riiber C. N. Das directe Ueberführen der Zimmtsäure in α-Truxillsäure. Ber. Dtsch. Chem. Ges. 1902, 35, 2908–2909. 10.1002/cber.19020350373. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

