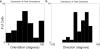Anatomy and function of an excitatory network in the visual cortex
- PMID: 27018655
- PMCID: PMC4844839
- DOI: 10.1038/nature17192
Anatomy and function of an excitatory network in the visual cortex
Abstract
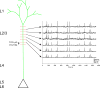
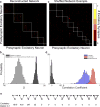
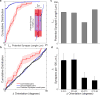
Circuits in the cerebral cortex consist of thousands of neurons connected by millions of synapses. A precise understanding of these local networks requires relating circuit activity with the underlying network structure. For pyramidal cells in superficial mouse visual cortex (V1), a consensus is emerging that neurons with similar visual response properties excite each other, but the anatomical basis of this recurrent synaptic network is unknown. Here we combined physiological imaging and large-scale electron microscopy to study an excitatory network in V1. We found that layer 2/3 neurons organized into subnetworks defined by anatomical connectivity, with more connections within than between groups. More specifically, we found that pyramidal neurons with similar orientation selectivity preferentially formed synapses with each other, despite the fact that axons and dendrites of all orientation selectivities pass near (<5 μm) each other with roughly equal probability. Therefore, we predict that mechanisms of functionally specific connectivity take place at the length scale of spines. Neurons with similar orientation tuning formed larger synapses, potentially enhancing the net effect of synaptic specificity. With the ability to study thousands of connections in a single circuit, functional connectomics is proving a powerful method to uncover the organizational logic of cortical networks.
Conflict of interest statement
The authors declare no competing financial interests.
Figures