Copper-catalysed enantioselective stereodivergent synthesis of amino alcohols
- PMID: 27018656
- PMCID: PMC4844805
- DOI: 10.1038/nature17191
Copper-catalysed enantioselective stereodivergent synthesis of amino alcohols
Abstract
The chirality, or 'handedness', of a biologically active molecule can alter its physiological properties. Thus it is routine procedure in the drug discovery and development process to prepare and fully characterize all possible stereoisomers of a drug candidate for biological evaluation. Despite many advances in asymmetric synthesis, developing general and practical strategies for obtaining all possible stereoisomers of an organic compound that has multiple contiguous stereocentres remains a challenge. Here, we report a stereodivergent copper-based approach for the expeditious construction of amino alcohols with high levels of chemo-, regio-, diastereo- and enantioselectivity. Specifically, we synthesized these amino-alcohol products using sequential, copper-hydride-catalysed hydrosilylation and hydroamination of readily available enals and enones. This strategy provides a route to all possible stereoisomers of the amino-alcohol products, which contain up to three contiguous stereocentres. We leveraged catalyst control and stereospecificity simultaneously to attain exceptional control of the product stereochemistry. Beyond the immediate utility of this protocol, our strategy could inspire the development of methods that provide complete sets of stereoisomers for other valuable synthetic targets.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




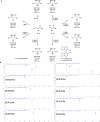
References
-
- Jozwiak K, Lough WJ, Wainer IW. Drug Stereochemistry: Analytical Methods and Pharmacology. Third. Informa; New York: 2012.
-
- Wermuth CG. The Practice of Medicinal Chemistry. Third. Elsevier; New York: 2008.
-
- Jacobsen EN, Pfaltz A, Yamamoto H, editors. Comprehensive Asymmetric Catalysis: Vol I–III, Suppl I–II. Springer; New York: 1999.
-
- Tian X, et al. Diastereodivergent asymmetric sulfa-Michael additions of α-branched enones using a single chiral organic catalyst. J Am Chem Soc. 2011;133:17934–17941. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

