Surface Chemistry and Microtopography of Parylene C Films Control the Morphology and Microtubule Density of Cardiac Myocytes
- PMID: 27018760
- PMCID: PMC4870650
- DOI: 10.1089/ten.TEC.2015.0581
Surface Chemistry and Microtopography of Parylene C Films Control the Morphology and Microtubule Density of Cardiac Myocytes
Abstract
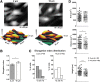
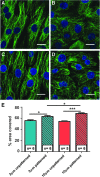
Cell micropatterning has certainly proved to improve the morphological and physiological properties of cardiomyocytes in vitro; however, there is little knowledge on the single cell-scaffold interactions that influence the cells' development and differentiation in culture. In this study, we employ hydrophobic/hydrophilic micropatterned Parylene C thin films (2-10 μm) as cell microscaffolds that can control the morphology and microtubule density of neonatal rat ventricular myocytes (NRVM) by regulating their adhesion area on Parylene through a thickness-dependent hydrophobicity. Structured NRVM on thin films tend to bridge across the hydrophobic areas, demonstrating a more spread-out shape and sparser microtubule organization, while cells on thicker films adopt a cylindrical (in vivo-like) shape (contact angles at the level of the nucleus are 64.51° and 84.73°, respectively) and a significantly (p < 0.05) denser microtubule structure. Ion scanning microscopy on NRVM revealed that cells on thicker membranes were significantly (p < 0.05) smaller in volume, but more elongated. The cylindrical shape and a significantly denser microtubule structure indicate the ability to influence cardiomyocyte phenotype using patterning and manipulation of hydrophilicity. These combined bioengineering strategies are promising tools in the generation of more representative cardiomyocytes in culture.
Figures



References
-
- Trantidou T., et al. . Selective hydrophilic modification of Parylene C films: a new approach to cell micro-patterning for synthetic biology applications. Biofabrication 6, 1, 2014 - PubMed
-
- Walsh K.B., and Parks G.E. Changes in cardiac myocyte morphology alter the properties of voltage-gated ion channels. Cardiovasc Res 55, 64, 2002 - PubMed
-
- Bien H., Yin L., and Entcheva E. Cardiac cell networks on elastic microgrooved scaffolds. IEEE Eng Med Biol Mag 22, 108, 2003 - PubMed
-
- Bursac N., Parker K., Iravanian S., and Tung L. Cardiomyocyte cultures with controlled macroscopic anisotropy a model for functional electrophysiological studies of cardiac muscle. Circ Res 91, e45, 2002 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

