Enabling Multiphoton and Second Harmonic Generation Imaging in Paraffin-Embedded and Histologically Stained Sections
- PMID: 27018844
- PMCID: PMC4922008
- DOI: 10.1089/ten.TEC.2016.0071
Enabling Multiphoton and Second Harmonic Generation Imaging in Paraffin-Embedded and Histologically Stained Sections
Abstract
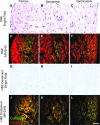
Nonlinear microscopy, namely multiphoton imaging and second harmonic generation (SHG), is an established noninvasive technique useful for the imaging of extracellular matrix (ECM). Typically, measurements are performed in vivo on freshly excised tissues or biopsies. In this article, we describe the effect of rehydrating paraffin-embedded sections on multiphoton and SHG emission signals and the acquisition of nonlinear images from hematoxylin and eosin (H&E)-stained sections before and after a destaining protocol. Our results reveal that bringing tissue sections to a physiological state yields a significant improvement in nonlinear signals, particularly in SHG. Additionally, the destaining of sections previously processed with H&E staining significantly improves their SHG emission signals during imaging, thereby allowing sufficient analysis of collagen in these sections. These results are important for researchers and pathologists to obtain additional information from paraffin-embedded tissues and archived samples to perform retrospective analysis of the ECM or gain additional information from rare samples.
Figures



References
-
- Ait El Madani H., Tancrède-Bohin E., Bensussan A., Colonna A., Dupuy A., Bagot M., and Pena A.M. In vivo multiphoton imaging of human skin: assessment of topical corticosteroid-induced epidermis atrophy and depigmentation. J Biomed Opt 17, 026009, 2012 - PubMed
-
- Vielreicher M., Schürmann S., Detsch R., Schmidt M.A., Buttgereit A., Boccaccini A., and Friedrich O. Taking a deep look: modern microscopy technologies to optimize the design and functionality of biocompatible scaffolds for tissue engineering in regenerative medicine. J R Soc Interface 10, 20130263, 2013 - PMC - PubMed
-
- Basuki J.S., Duong H.T., Macmillan A., Erlich R.B., Esser L., Akerfeldt M.C., Whan R.M., Kavallaris M., Boyer C., and Davis T.P. Using fluorescence lifetime imaging microscopy to monitor theranostic nanoparticle uptake and intracellular doxorubicin release. ACS Nano 7, 10175, 2013 - PubMed
-
- Fritze O., Schleicher M., König K., Schenke-Layland K., Stock U., and Harasztosi C. Facilitated noninvasive visualization of collagen and elastin in blood vessels. Tissue Eng Part C Methods 16, 705, 2010 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
