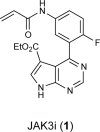
Essential biphasic role for JAK3 catalytic activity in IL-2 receptor signaling
- PMID: 27018889
- PMCID: PMC4837022
- DOI: 10.1038/nchembio.2056
Essential biphasic role for JAK3 catalytic activity in IL-2 receptor signaling
Abstract
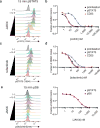
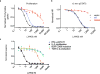
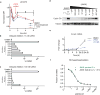
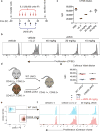
To drive lymphocyte proliferation and differentiation, common γ-chain (γc) cytokine receptors require hours to days of sustained stimulation. JAK1 and JAK3 kinases are found together in all γc-receptor complexes, but how their respective catalytic activities contribute to signaling over time is not known. Here we dissect the temporal requirements for JAK3 kinase activity with a selective covalent inhibitor (JAK3i). By monitoring phosphorylation of the transcription factor STAT5 over 20 h in CD4(+) T cells stimulated with interleukin 2 (IL-2), we document a second wave of signaling that is much more sensitive to JAK3i than the first wave. Selective inhibition of this second wave is sufficient to block cyclin expression and entry to S phase. An inhibitor-resistant JAK3 mutant (C905S) rescued all effects of JAK3i in isolated T cells and in mice. Our chemical genetic toolkit elucidates a biphasic requirement for JAK3 kinase activity in IL-2-driven T cell proliferation and will find broad utility in studies of γc-receptor signaling.
Conflict of interest statement
None.
Figures
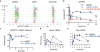
Comment in
-
Immunology: JAK3 inhibition—is it sufficient?Nat Chem Biol. 2016 May;12(5):308-10. doi: 10.1038/nchembio.2066. Nat Chem Biol. 2016. PMID: 27556128 No abstract available.
Similar articles
-
Jak1 has a dominant role over Jak3 in signal transduction through γc-containing cytokine receptors.Chem Biol. 2011 Mar 25;18(3):314-23. doi: 10.1016/j.chembiol.2011.01.012. Chem Biol. 2011. PMID: 21439476
-
Delineation of the regions of interleukin-2 (IL-2) receptor beta chain important for association of Jak1 and Jak3. Jak1-independent functional recruitment of Jak3 to Il-2Rbeta.J Biol Chem. 1998 Apr 24;273(17):10719-25. doi: 10.1074/jbc.273.17.10719. J Biol Chem. 1998. PMID: 9553136
-
Activation of JAK3, but not JAK1, is critical for IL-2-induced proliferation and STAT5 recruitment by a COOH-terminal region of the IL-2 receptor beta-chain.Cytokine. 1995 Oct;7(7):689-700. doi: 10.1006/cyto.1995.0081. Cytokine. 1995. PMID: 8580378
-
Signalling by cytokines interacting with the interleukin-2 receptor gamma chain.Cytokines Cell Mol Ther. 1998 Dec;4(4):243-56. Cytokines Cell Mol Ther. 1998. PMID: 10068058 Review.
-
JAK3: a two-faced player in hematological disorders.Int J Biochem Cell Biol. 2009 Dec;41(12):2376-9. doi: 10.1016/j.biocel.2009.09.004. Epub 2009 Sep 9. Int J Biochem Cell Biol. 2009. PMID: 19747563 Free PMC article. Review.
Cited by
-
Cbl-b mitigates the responsiveness of naive CD8+ T cells that experience extensive tonic T cell receptor signaling.Sci Signal. 2024 Feb 6;17(822):eadh0439. doi: 10.1126/scisignal.adh0439. Epub 2024 Feb 6. Sci Signal. 2024. PMID: 38319998 Free PMC article.
-
Breakthrough of solid tumor treatment: CAR-NK immunotherapy.Cell Death Discov. 2024 Jan 20;10(1):40. doi: 10.1038/s41420-024-01815-9. Cell Death Discov. 2024. PMID: 38245520 Free PMC article. Review.
-
Generation of a chemical genetic model for JAK3.Sci Rep. 2021 May 12;11(1):10093. doi: 10.1038/s41598-021-89356-4. Sci Rep. 2021. PMID: 33980892 Free PMC article.
-
Cutting Edge: IL-2-Induced Expression of the Amino Acid Transporters SLC1A5 and CD98 Is a Prerequisite for NKG2D-Mediated Activation of Human NK Cells.J Immunol. 2017 Sep 15;199(6):1967-1972. doi: 10.4049/jimmunol.1700497. Epub 2017 Aug 7. J Immunol. 2017. PMID: 28784848 Free PMC article.
-
Disorders of the JAK/STAT Pathway in T Cell Lymphoma Pathogenesis: Implications for Immunotherapy.Annu Rev Immunol. 2017 Apr 26;35:533-550. doi: 10.1146/annurev-immunol-110416-120628. Epub 2017 Feb 9. Annu Rev Immunol. 2017. PMID: 28182501 Free PMC article. Review.
References
-
- Miyazaki T, et al. Functional activation of Jak1 and Jak3 by selective association with IL-2 receptor subunits. Science. 1994;266:1045–1047. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

