GABAergic signaling in the rat pineal gland
- PMID: 27019076
- PMCID: PMC5489258
- DOI: 10.1111/jpi.12328
GABAergic signaling in the rat pineal gland
Abstract
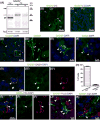
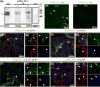
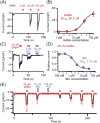
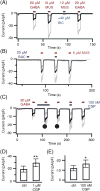
Pinealocytes secrete melatonin at night in response to norepinephrine released from sympathetic nerve terminals in the pineal gland. The gland also contains many other neurotransmitters whose cellular disposition, activity, and relevance to pineal function are not understood. Here, we clarify sources and demonstrate cellular actions of the neurotransmitter γ-aminobutyric acid (GABA) using Western blotting and immunohistochemistry of the gland and electrical recording from pinealocytes. GABAergic cells and nerve fibers, defined as containing GABA and the synthetic GAD67, were identified. The cells represent a subset of interstitial cells while the nerve fibers were distinct from the sympathetic innervation. The GABAA receptor subunit α1 was visualized in close proximity of both GABAergic and sympathetic nerve fibers as well as fine extensions among pinealocytes and blood vessels. The GABAB 1 receptor subunit was localized in the interstitial compartment but not in pinealocytes. Electrophysiology of isolated pinealocytes revealed that GABA and muscimol elicit strong inward chloride currents sensitive to bicuculline and picrotoxin, clear evidence for functional GABAA receptors on the surface membrane. Applications of elevated potassium solution or the neurotransmitter acetylcholine depolarized the pinealocyte membrane potential enough to open voltage-gated Ca(2+) channels leading to intracellular calcium elevations. GABA repolarized the membrane and shut off such calcium rises. In 48-72-h cultured intact glands, GABA application neither triggered melatonin secretion by itself nor affected norepinephrine-induced secretion. Thus, strong elements of GABA signaling are present in pineal glands that make large electrical responses in pinealocytes, but physiological roles need to be found.
Keywords: GABA; GABAA receptor; GAD67; bicuculline; melatonin secretion; membrane potential; pineal gland.
© 2016 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Figures

Similar articles
-
Melatonin inhibits voltage-gated potassium KV4.2 channels and negatively regulates melatonin secretion in rat pineal glands.Am J Physiol Cell Physiol. 2024 Oct 1;327(4):C1023-C1034. doi: 10.1152/ajpcell.00664.2023. Epub 2024 Aug 19. Am J Physiol Cell Physiol. 2024. PMID: 39159388
-
Central GABAergic innervation of the mammalian pineal gland: a light and electron microscopic immunocytochemical investigation in rodent and nonrodent species.J Comp Neurol. 2001 Jan 29;430(1):72-84. doi: 10.1002/1096-9861(20010129)430:1<72::aid-cne1015>3.0.co;2-t. J Comp Neurol. 2001. PMID: 11135246
-
TMEM16A and TMEM16B channel proteins generate Ca2+-activated Cl- current and regulate melatonin secretion in rat pineal glands.J Biol Chem. 2018 Jan 19;293(3):995-1006. doi: 10.1074/jbc.RA117.000326. Epub 2017 Nov 29. J Biol Chem. 2018. PMID: 29187602 Free PMC article.
-
GABA as a presumptive paracrine signal in the pineal gland. Evidence on an intrapineal GABAergic system.Brain Res Bull. 1990 Aug;25(2):339-44. doi: 10.1016/0361-9230(90)90080-j. Brain Res Bull. 1990. PMID: 2171722 Review.
-
Cellular and molecular mechanisms controlling melatonin release by mammalian pineal glands.Cell Mol Neurobiol. 1987 Dec;7(4):323-37. doi: 10.1007/BF00733786. Cell Mol Neurobiol. 1987. PMID: 2897878 Free PMC article. Review.
Cited by
-
Serotonin modulates melatonin synthesis as an autocrine neurotransmitter in the pineal gland.Proc Natl Acad Sci U S A. 2021 Oct 26;118(43):e2113852118. doi: 10.1073/pnas.2113852118. Proc Natl Acad Sci U S A. 2021. PMID: 34675083 Free PMC article.
-
Impact of aging on the GABAB receptor-mediated connectome.bioRxiv [Preprint]. 2024 Aug 2:2024.07.31.606013. doi: 10.1101/2024.07.31.606013. bioRxiv. 2024. PMID: 39131332 Free PMC article. Preprint.
-
Sedative-Hypnotic Activity of the Water Extracts of Coptidis Rhizoma in Rodents.Clocks Sleep. 2022 Mar 4;4(1):145-159. doi: 10.3390/clockssleep4010014. Clocks Sleep. 2022. PMID: 35323168 Free PMC article.
-
Cellular Basis of Pineal Gland Development: Emerging Role of Microglia as Phenotype Regulator.PLoS One. 2016 Nov 18;11(11):e0167063. doi: 10.1371/journal.pone.0167063. eCollection 2016. PLoS One. 2016. PMID: 27861587 Free PMC article.
-
Safety Challenges of Using High Dose Baclofen for Alcohol Use Disorder: A Focused Review.Front Psychiatry. 2018 Aug 22;9:367. doi: 10.3389/fpsyt.2018.00367. eCollection 2018. Front Psychiatry. 2018. PMID: 30186187 Free PMC article. Review.
References
-
- MØLLER M. Fine structure of the pinealopetal innervation of the mammalian pineal gland. Microsc Res Tech. 1992;21:188–204. - PubMed
-
- SIMONNEAUX V, RIBELAYGA C. Generation of the melatonin endocrine message in mammals: a review of the complex regulation of melatonin synthesis by norepinephrine, peptides, and other pineal transmitters. Pharmacol Rev. 2003;55:325–395. - PubMed
-
- KLEIN DC. Arylalkylamine N-acetyltransferase: “the Timezyme”. J Biol Chem. 2007;282:4233–4237. - PubMed
-
- KLEIN DC, COON SL, ROSEBOOM PH, et al. The melatonin rhythm-generating enzyme: molecular regulation of serotonin N-acetyltransferase in the pineal gland. Recent Prog Horm Res. 1997;52:307–357. discussion 357–358. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

