Long Non-Coding RNA MALAT1 Mediates Transforming Growth Factor Beta1-Induced Epithelial-Mesenchymal Transition of Retinal Pigment Epithelial Cells
- PMID: 27019196
- PMCID: PMC4809592
- DOI: 10.1371/journal.pone.0152687
Long Non-Coding RNA MALAT1 Mediates Transforming Growth Factor Beta1-Induced Epithelial-Mesenchymal Transition of Retinal Pigment Epithelial Cells
Abstract
Purpose: To study the role of long non-coding RNA (lncRNA) MALAT1 in transforming growth factor beta 1 (TGF-β1)-induced epithelial-mesenchymal transition (EMT) of retinal pigment epithelial (RPE) cells.
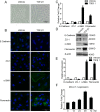
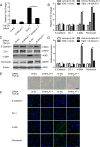
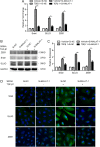
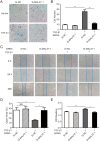
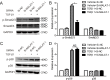
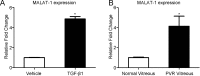
Methods: ARPE-19 cells were cultured and exposed to TGF-β1. The EMT of APRE-19 cells is confirmed by morphological change, as well as the increased expression of alpha-smooth muscle actin (αSMA) and fibronectin, and the down-regulation of E-cadherin and Zona occludin-1(ZO-1) at both mRNA and protein levels. The expression of lncRNA MALAT1 in RPE cells were detected by quantitative real-time PCR. Knockdown of MALAT1 was achieved by transfecting a small interfering RNA (SiRNA). The effect of inhibition of MALAT1 on EMT, migration, proliferation, and TGFβ signalings were observed. MALAT1 expression was also detected in primary RPE cells incubated with proliferative vitreoretinopathy (PVR) vitreous samples.
Results: The expression of MALAT1 is significantly increased in RPE cells incubated with TGFβ1. MALAT1 silencing attenuates TGFβ1-induced EMT, migration, and proliferation of RPE cells, at least partially through activating Smad2/3 signaling. MALAT1 is also significantly increased in primary RPE cells incubated with PVR vitreous samples.
Conclusion: LncRNA MALAT1 is involved in TGFβ1-induced EMT of human RPE cells and provides new understandings for the pathogenesis of PVR.
Conflict of interest statement
Figures
Similar articles
-
Protective Effects of Fucoidan on Epithelial-Mesenchymal Transition of Retinal Pigment Epithelial Cells and Progression of Proliferative Vitreoretinopathy.Cell Physiol Biochem. 2018;46(4):1704-1715. doi: 10.1159/000489246. Epub 2018 Apr 23. Cell Physiol Biochem. 2018. PMID: 29698960
-
Snail involves in the transforming growth factor β1-mediated epithelial-mesenchymal transition of retinal pigment epithelial cells.PLoS One. 2011;6(8):e23322. doi: 10.1371/journal.pone.0023322. Epub 2011 Aug 10. PLoS One. 2011. PMID: 21853110 Free PMC article.
-
Overexpression of Snail in retinal pigment epithelial triggered epithelial-mesenchymal transition.Biochem Biophys Res Commun. 2014 Mar 28;446(1):347-51. doi: 10.1016/j.bbrc.2014.02.119. Epub 2014 Mar 4. Biochem Biophys Res Commun. 2014. PMID: 24607896
-
Blockade of Jagged/Notch pathway abrogates transforming growth factor β2-induced epithelial-mesenchymal transition in human retinal pigment epithelium cells.Curr Mol Med. 2014 May;14(4):523-34. doi: 10.2174/1566524014666140331230411. Curr Mol Med. 2014. PMID: 24694299 Review.
-
The intervention of epithelial-mesenchymal transition in homeostasis of human retinal pigment epithelial cells: a review.J Histotechnol. 2022 Dec;45(4):148-160. doi: 10.1080/01478885.2022.2137665. Epub 2022 Nov 15. J Histotechnol. 2022. PMID: 36377481 Review.
Cited by
-
Network analysis of long non-coding RNA expression profiles in common warts.Heliyon. 2022 Nov 19;8(11):e11790. doi: 10.1016/j.heliyon.2022.e11790. eCollection 2022 Nov. Heliyon. 2022. PMID: 36458289 Free PMC article.
-
Inflammatory mediators of proliferative vitreoretinopathy: hypothesis and review.Int Ophthalmol. 2020 Jun;40(6):1587-1601. doi: 10.1007/s10792-020-01325-4. Epub 2020 Feb 26. Int Ophthalmol. 2020. PMID: 32103371 Free PMC article. Review.
-
Effects of bradykinin on TGF‑β1‑induced epithelial‑mesenchymal transition in ARPE‑19 cells.Mol Med Rep. 2018 Apr;17(4):5878-5886. doi: 10.3892/mmr.2018.8556. Epub 2018 Feb 2. Mol Med Rep. 2018. PMID: 29436636 Free PMC article.
-
Polarity and epithelial-mesenchymal transition of retinal pigment epithelial cells in proliferative vitreoretinopathy.PeerJ. 2020 Oct 20;8:e10136. doi: 10.7717/peerj.10136. eCollection 2020. PeerJ. 2020. PMID: 33150072 Free PMC article.
-
Extracellular Vesicles Released by Tumor Endothelial Cells Spread Immunosuppressive and Transforming Signals Through Various Recipient Cells.Front Cell Dev Biol. 2020 Sep 9;8:698. doi: 10.3389/fcell.2020.00698. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33015029 Free PMC article.
References
-
- Kim IK, Arroyo JG (2002) Mechanisms in proliferative vitreoretinopathy. Ophthalmol Clin North Am 15: 81–86. - PubMed
-
- Cardillo JA, Stout JT, LaBree L, Azen SP, Omphroy L, Cui JZ, et al. (1997) Post-traumatic Proliferative Vitreoretinopathy: The Epidemiologfic Profile, Onset, Risk Factors, and Visual Outcome. Ophthalmology 104: 1166–1173. - PubMed
-
- Machemer R, van Horn D, Aaberg TM (1978) Pigment epithelial proliferation in human retinal detachment with massive periretinal proliferation. American journal of ophthalmology 85: 181–191. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

