Luffa echinata Roxb. Induced Apoptosis in Human Colon Cancer Cell (SW-480) in the Caspase-dependent Manner and Through a Mitochondrial Apoptosis Pathway
- PMID: 27019558
- PMCID: PMC4787332
- DOI: 10.4103/0973-1296.176017
Luffa echinata Roxb. Induced Apoptosis in Human Colon Cancer Cell (SW-480) in the Caspase-dependent Manner and Through a Mitochondrial Apoptosis Pathway
Abstract
Background: Luffa echinata Roxb. (LER) (Cucurbitaceae) showed tremendous medicinal importance and are being used for the treatment of different ailments.
Objective: In this study, the antiproliferative properties and cell death mechanism induced by the extract of the fruits of LER were investigated.
Materials and methods: MTT and LDH assay were used to test the antiproliferative and cytotoxicity of LER extract, respectively. The intracellular ROS were measured by a fluorometric assay. The expression of several apoptotic-related proteins in SW-480 cells treated by LER was evaluated by Western blot analysis.
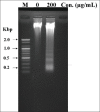
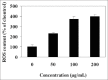
Results: The methanolic extract of LER fruits inhibited the proliferation of human colon cancer cells (SW-480) in both dose- and time-dependent manners. The LER-treated cells showed obvious characteristics of cell apoptosis, including cell shrinkage, destruction of the monolayer, and condensed chromatin. In addition, treatments of various concentrations of LER extracts caused the release of lactate dehydrogenase as a dose-dependent manner via stimulation of the intracellular metabolic system. LER induced apoptosis, DNA fragmentation, and cellular ROS accumulation in SW-480 cells. Treatment of LER on SW-480 cells promoted the expression of caspases, Bax, Bad, and p53 proteins and decreased the levels of Bcl-2 and Bcl-XL.
Conclusions: These results indicated that treatment with LER-induced cell death in mitochondrial apoptosis pathway by regulating pro-apoptotic proteins via the up regulation of the p53 protein. These findings highlight the potentials of LER in the treatment of human colon cancer.
Summary: LER induced apoptosis, DNA fragmentation, and cellular ROS accumulation in SW-480 cells. Treatment of LER on SW-480 cells promoted the expression of caspases, Bax, Bad, and p53 proteins and decreased the levels of Bcl-2 and Bcl-XL.
Keywords: Apoptosis; Luffa echinata Roxb For the treated cells, viability was calculated; caspases; colon cancer cells; p53.
Figures






Similar articles
-
Luffa echinata Roxb. induces human colon cancer cell (HT-29) death by triggering the mitochondrial apoptosis pathway.Molecules. 2012 May 16;17(5):5780-94. doi: 10.3390/molecules17055780. Molecules. 2012. PMID: 22592084 Free PMC article.
-
An anthraquinone derivative from Luffa acutangula induces apoptosis in human lung cancer cell line NCI-H460 through p53-dependent pathway.J Recept Signal Transduct Res. 2016;36(3):292-302. doi: 10.3109/10799893.2015.1108335. Epub 2015 Nov 20. J Recept Signal Transduct Res. 2016. PMID: 26585176
-
Inhibitory effect of Curcuma purpurascens BI. rhizome on HT-29 colon cancer cells through mitochondrial-dependent apoptosis pathway.BMC Complement Altern Med. 2015 Feb 5;15:15. doi: 10.1186/s12906-015-0534-6. BMC Complement Altern Med. 2015. PMID: 25652758 Free PMC article.
-
Elephantopus scaber induces apoptosis through ROS-dependent mitochondrial signaling pathway in HCT116 human colorectal carcinoma cells.J Ethnopharmacol. 2015 Jun 20;168:291-304. doi: 10.1016/j.jep.2015.03.072. Epub 2015 Apr 8. J Ethnopharmacol. 2015. PMID: 25861953
-
Viscum articulatum Burm. f. aqueous extract exerts antiproliferative effect and induces cell cycle arrest and apoptosis in leukemia cells.J Ethnopharmacol. 2018 Jun 12;219:91-102. doi: 10.1016/j.jep.2018.03.005. Epub 2018 Mar 16. J Ethnopharmacol. 2018. PMID: 29555410
Cited by
-
Comparison of Phytochemical Composition and Untargeted Metabolomic Analysis of an Extract from Cnidoscolus aconitifolius (Mill.) I. I. Johnst and Porophyllum ruderale (Jacq.) Cass. and Biological Cytotoxic and Antiproliferative Activity In Vitro.Plants (Basel). 2023 May 15;12(10):1987. doi: 10.3390/plants12101987. Plants (Basel). 2023. PMID: 37653904 Free PMC article.
References
-
- Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–17. - PubMed
-
- Rasool S, Kadla SA, Rasool V, Ganai BA. A comparative overview of general risk factors associated with the incidence of colorectal cancer. Tumour Biol. 2013;34:2469–76. - PubMed
-
- Ahnen DJ, Wade SW, Jones WF, Sifri R, Mendoza Silveiras J, Greenamyer J, et al. The increasing incidence of young-onset colorectal cancer: A call to action. Mayo Clin Proc. 2014;89:216–24. - PubMed
-
- Chastek B, Kulakodlu M, Valluri S, Seal B. Impact of metastatic colorectal cancer stage and number of treatment courses on patient health care costs and utilization. Postgrad Med. 2013;125:73–82. - PubMed
-
- Urruticoechea A, Alemany R, Balart J, Villanueva A, Viñals F, Capellá G. Recent advances in cancer therapy: An overview. Curr Pharm Des. 2010;16:3–10. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
