High-content screening identifies a role for Na(+) channels in insulin production
- PMID: 27019722
- PMCID: PMC4807443
- DOI: 10.1098/rsos.150306
High-content screening identifies a role for Na(+) channels in insulin production
Abstract
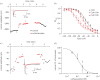
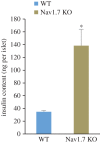
Insulin production is the central feature of functionally mature and differentiated pancreatic β-cells. Reduced insulin transcription and dedifferentiation have been implicated in type 2 diabetes, making drugs that could reverse these processes potentially useful. We have previously established ratiometric live-cell imaging tools to identify factors that increase insulin promoter activity and promote β-cell differentiation. Here, we present a single vector imaging tool with eGFP and mRFP, driven by the Pdx1 and Ins1 promoters, respectively, targeted to the nucleus to enhance identification of individual cells in a high-throughput manner. Using this new approach, we screened 1120 off-patent drugs for factors that regulate Ins1 and Pdx1 promoter activity in MIN6 β-cells. We identified a number of compounds that positively modulate Ins1 promoter activity, including several drugs known to modulate ion channels. Carbamazepine was selected for extended follow-up, as our previous screen also identified this use-dependent sodium channel inhibitor as a positive modulator of β-cell survival. Indeed, carbamazepine increased Ins1 and Ins2 mRNA in primary mouse islets at lower doses than were required to protect β-cells. We validated the role of sodium channels in insulin production by examining Nav1.7 (Scn9a) knockout mice and remarkably islets from these animals had dramatically elevated insulin content relative to wild-type controls. Collectively, our experiments provide a starting point for additional studies aimed to identify drugs and molecular pathways that control insulin production and β-cell differentiation status. In particular, our unbiased screen identified a novel role for a β-cell sodium channel gene in insulin production.
Keywords: high-content screening; insulin synthesis; islet beta-cells; live-cell imaging; sodium channels.
Figures
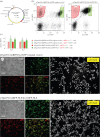

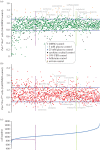
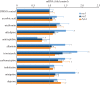
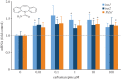
References
-
- Johnson JD, Luciani DS. 2010. Mechanisms of pancreatic beta-cell apoptosis in diabetes and its therapies. Adv. Exp. Med. Biol. 654, 447–462. (doi:10.1007/978-90-481-3271-3_19) - DOI - PubMed
-
- Prentki M, Nolan CJ. 2006. Islet beta cell failure in type 2 diabetes. J. Clin. Invest. 116, 1802–1812. (doi:10.1172/JCI29103) - DOI - PMC - PubMed
-
- Mehran AE, et al. 2012. Hyperinsulinemia drives diet-induced obesity independently of brain insulin production. Cell Metab. 16, 723–737. (doi:10.1016/j.cmet.2012.10.019) - DOI - PubMed
-
- Rezania A, et al. 2014. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat. Biotechnol. 32, 1121–1133. (doi:10.1038/nbt.3033) - DOI - PubMed
-
- Szabat M, Johnson JD, Piret JM. 2010. Reciprocal modulation of adult beta cell maturity by activin A and follistatin. Diabetologia 53, 1680–1689. (doi:10.1007/s00125-010-1758-0) - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
