Loss of MYO5B in mice recapitulates Microvillus Inclusion Disease and reveals an apical trafficking pathway distinct to neonatal duodenum
- PMID: 27019864
- PMCID: PMC4806369
- DOI: 10.1016/j.jcmgh.2015.11.009
Loss of MYO5B in mice recapitulates Microvillus Inclusion Disease and reveals an apical trafficking pathway distinct to neonatal duodenum
Abstract
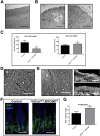
Background and aims: Inactivating mutations in MYO5B cause severe neonatal diarrhea in Microvillus Inclusion Disease. Loss of active MYO5B causes the formation of pathognomonic inclusions and aberrations in brush border enzymes.
Methods: We developed three mouse models of germline, constitutively intestinal targeted and inducible intestinal targeted deletion of MYO5B. The mice were evaluated for enterocyte cellular morphology.
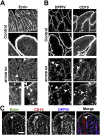
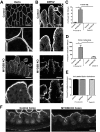
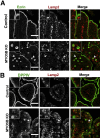
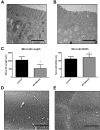
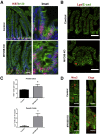
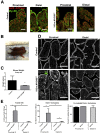
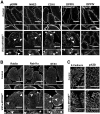
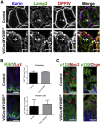
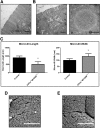
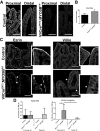
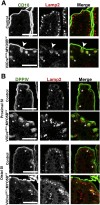
Results: Germline MYO5B KO mice showed early diarrhea and failure to thrive with evident microvillus inclusions and loss of apical transporters in the duodenum. IgG was present within inclusions. Apical transporters were lost and inclusions were present in the duodenum, but were nearly absent in the ileum. VillinCre;MYO5BF/F mice showed similar pathology and morphological changes in duodenal enterocytes. In contrast, when MYO5B KO was induced with tamoxifen treatment at 8 weeks of age, VillinCreERT2;MYO5BF/F mice developed severe diarrhea with loss of duodenal brush border enzymes, but few inclusions were observed in enterocytes. However, if tamoxifen is administered to 2-day-old VillinCreERT2;MYO5BF/F mice, prominent microvillus inclusions were observed.
Conclusions: The microvillus inclusions that develop after MYO5B loss reveal the presence of an unrecognized apical membrane trafficking pathway in neonatal duodenal enterocytes. However, the diarrheal pathology after MYO5B loss is due to deficits in transporter presentation at the apical membrane in duodenal enterocytes.
Keywords: Enterocyte trafficking; NHE3; Rab11a; Rab8a; Syntaxin 3; brush border.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures








References
-
- Erickson R.P., Larson-Thome K., Valenzuela R.K. Navajo microvillous inclusion disease is due to a mutation in MYO5B. Am J Med Genet A. 2008;146A:3117–3119. - PubMed
-
- Muller T., Hess M.W., Schiefermeier N. MYO5B mutations cause microvillus inclusion disease and disrupt epithelial cell polarity. Nat Genet. 2008;40:1163–1165. - PubMed
-
- Ruemmele F.M., Muller T., Schiefermeier N. Loss-of-function of MYO5B is the main cause of microvillus inclusion disease: 15 novel mutations and a CaCo-2 RNAi cell model. Hum Mutat. 2010;31:544–551. - PubMed
-
- Davidson G.P., Cutz E., Hamilton J.R. Familial enteropathy: a syndrome of protracted diarrhea from birth, failure to thrive, and hypoplastic villus atrophy. Gastroenterology. 1978;75:783–790. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
