LUMiC® Endoprosthetic Reconstruction After Periacetabular Tumor Resection: Short-term Results
- PMID: 27020434
- PMCID: PMC5289170
- DOI: 10.1007/s11999-016-4805-4
LUMiC® Endoprosthetic Reconstruction After Periacetabular Tumor Resection: Short-term Results
Abstract
Background: Reconstruction of periacetabular defects after pelvic tumor resection ranks among the most challenging procedures in orthopaedic oncology, and reconstructive techniques are generally associated with dissatisfying mechanical and nonmechanical complication rates. In an attempt to reduce the risk of dislocation, aseptic loosening, and infection, we introduced the LUMiC® prosthesis (implantcast, Buxtehude, Germany) in 2008. The LUMiC® prosthesis is a modular device, built of a separate stem (hydroxyapatite-coated uncemented or cemented) and acetabular cup. The stem and cup are available in different sizes (the latter of which is also available with silver coating for infection prevention) and are equipped with sawteeth at the junction to allow for rotational adjustment of cup position after implantation of the stem. Whether this implant indeed is durable at short-term followup has not been evaluated.
Questions/purposes: (1) What proportion of patients experience mechanical complications and what are the associated risk factors of periacetabular reconstruction with the LUMiC® after pelvic tumor resection? (2) What proportion of patients experience nonmechanical complications and what are the associated risk factors of periacetabular reconstruction with the LUMiC® after pelvic tumor resection? (3) What is the cumulative incidence of implant failure at 2 and 5 years and what are the mechanisms of reconstruction failure? (4) What is the functional outcome as assessed by Musculoskeletal Tumor Society (MSTS) score at final followup?
Methods: We performed a retrospective chart review of every patient in whom a LUMiC® prosthesis was used to reconstruct a periacetabular defect after internal hemipelvectomy for a pelvic tumor from July 2008 to June 2014 in eight centers of orthopaedic oncology with a minimum followup of 24 months. Forty-seven patients (26 men [55%]) with a mean age of 50 years (range, 12-78 years) were included. At review, 32 patients (68%) were alive. The reverse Kaplan-Meier method was used to calculate median followup, which was equal to 3.9 years (95% confidence interval [CI], 3.4-4.3). During the period under study, our general indications for using this implant were reconstruction of periacetabular defects after pelvic tumor resections in which the medial ilium adjacent to the sacroiliac joint was preserved; alternative treatments included hip transposition and saddle or custom-made prostheses in some of the contributing centers; these were generally used when the medial ilium was involved in the tumorous process or if the LUMiC® was not yet available in the specific country at that time. Conventional chondrosarcoma was the predominant diagnosis (n = 22 [47%]); five patients (11%) had osseous metastases of a distant carcinoma and three (6%) had multiple myeloma. Uncemented fixation (n = 43 [91%]) was preferred. Dual-mobility cups (n = 24 [51%]) were mainly used in case of a higher presumed risk of dislocation in the early period of our study; later, dual-mobility cups became the standard for the majority of the reconstructions. Silver-coated acetabular cups were used in 29 reconstructions (62%); because only the largest cup size was available with silver coating, its use depended on the cup size that was chosen. We used a competing risk model to estimate the cumulative incidence of implant failure.
Results: Six patients (13%) had a single dislocation; four (9%) had recurrent dislocations. The risk of dislocation was lower in reconstructions with a dual-mobility cup (one of 24 [4%]) than in those without (nine of 23 [39%]) (hazard ratio, 0.11; 95% CI, 0.01-0.89; p = 0.038). Three patients (6%; one with a preceding structural allograft reconstruction, one with poor initial fixation as a result of an intraoperative fracture, and one with a cemented stem) had loosening and underwent revision. Infections occurred in 13 reconstructions (28%). Median duration of surgery was 6.5 hours (range, 4.0-13.6 hours) for patients with an infection and 5.3 hours (range, 2.8-9.9 hours) for those without (p = 0.060); blood loss was 2.3 L (range, 0.8-8.2 L) for patients with an infection and 1.5 L (range, 0.4-3.8 L) for those without (p = 0.039). The cumulative incidences of implant failure at 2 and 5 years were 2.1% (95% CI, 0-6.3) and 17.3% (95% CI, 0.7-33.9) for mechanical reasons and 6.4% (95% CI, 0-13.4) and 9.2% (95% CI, 0.5-17.9) for infection, respectively. Reasons for reconstruction failure were instability (n = 1 [2%]), loosening (n = 3 [6%]), and infection (n = 4 [9%]). Mean MSTS functional outcome score at followup was 70% (range, 33%-93%).
Conclusions: At short-term followup, the LUMiC® prosthesis demonstrated a low frequency of mechanical complications and failure when used to reconstruct the acetabulum in patients who underwent major pelvic tumor resections, and we believe this is a useful reconstruction for periacetabular resections for tumor or failed prior reconstructions. Still, infection and dislocation are relatively common after these complex reconstructions. Dual-mobility articulation in our experience is associated with a lower risk of dislocation. Future, larger studies will need to further control for factors such as dual-mobility articulation and silver coating. We will continue to follow our patients over the longer term to ascertain the role of this implant in this setting.
Level of evidence: Level IV, therapeutic study.
Figures
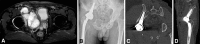
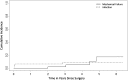
Comment in
-
CORR Insights®: LUMiC® Endoprosthetic Reconstruction After Periacetabular Tumor Resection: Short-term Results.Clin Orthop Relat Res. 2017 Mar;475(3):696-697. doi: 10.1007/s11999-016-4853-9. Epub 2016 Apr 26. Clin Orthop Relat Res. 2017. PMID: 27116207 Free PMC article. No abstract available.
References
-
- Aboulafia AJ, Buch R, Mathews J, Li W, Malawer MM. Reconstruction using the saddle prosthesis following excision of primary and metastatic periacetabular tumors. Clin Orthop Relat Res. 1995;314:203–213. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

