Compression therapy affects collagen type balance in hypertrophic scar
- PMID: 27020811
- PMCID: PMC4813311
- DOI: 10.1016/j.jss.2015.10.040
Compression therapy affects collagen type balance in hypertrophic scar
Abstract
Background: The effects of pressure on hypertrophic scar are poorly understood. Decreased extracellular matrix deposition is hypothesized to contribute to changes observed after pressure therapy. To examine this further, collagen composition was analyzed in a model of pressure therapy in hypertrophic scar.
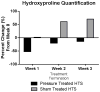
Materials and methods: Hypertrophic scars created on red Duroc swine (n = 8) received pressure treatment (pressure device mounting and delivery at 30 mm Hg), sham treatment (device mounting and no delivery), or no treatment for 2 wk. Scars were assessed weekly and biopsied for histology, hydroxyproline quantification, and gene expression analysis. Transcription levels of collagen precursors COL1A2 and COL3A1 were quantified using reverse transcription-polymerase chain reaction. Masson trichrome was used for general collagen quantification, whereas immunofluorescence was used for collagen types I and III specific quantification.
Results: Total collagen quantification using hydroxyproline assay showed a 51.9% decrease after pressure initiation. Masson trichrome staining showed less collagen after 1 (P < 0.03) and 2 wk (P < 0.002) of pressure application compared with sham and untreated scars. Collagen 1A2 and 3A1 transcript decreased by 41.9- and 42.3-fold, respectively, compared with uninjured skin after pressure treatment, whereas a 2.3- and 1.3-fold increase was seen in untreated scars. This decrease was seen in immunofluorescence staining for collagen types I (P < 0.001) and III (P < 0.04) compared with pretreated levels. Pressure-treated scars also had lower levels of collagen I and III after pressure treatment (P < 0.05) compared with sham and untreated scars.
Conclusions: These results demonstrate the modulation of collagen after pressure therapy and further characterize its role in scar formation and therapy.
Keywords: Burn scar; Collagen; Compression therapy; Hypertrophic scar; Pressure therapy.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures


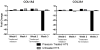




References
-
- Wolfram D, et al. Hypertrophic scars and keloids--a review of their pathophysiology, risk factors, and therapeutic management. Dermatol Surg. 2009;35(2):171–81. - PubMed
-
- Bell L, et al. Pruritus in burns: a descriptive study. J Burn Care Rehabil. 1988;9(3):305–8. - PubMed
-
- Slemp AE, Kirschner RE. Keloids and scars: a review of keloids and scars, their pathogenesis, risk factors, and management. Curr Opin Pediatr. 2006;18(4):396–402. - PubMed
-
- Robert R, et al. Disfiguring burn scars and adolescent self-esteem. Burns: journal of the International Society for Burn Injuries. 1999;25(7):581–5. - PubMed
-
- Taal L, Faber AW. Posttraumatic stress and maladjustment among adult burn survivors 1 to 2 years postburn Part II: the interview data. Burns. 1998;24(5):399–405. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

