Early Adaptation and Acquired Resistance to CDK4/6 Inhibition in Estrogen Receptor-Positive Breast Cancer
- PMID: 27020857
- PMCID: PMC5426059
- DOI: 10.1158/0008-5472.CAN-15-0728
Early Adaptation and Acquired Resistance to CDK4/6 Inhibition in Estrogen Receptor-Positive Breast Cancer
Erratum in
-
Correction: Early Adaptation and Acquired Resistance to CDK4/6 Inhibition in Estrogen Receptor-Positive Breast Cancer.Cancer Res. 2016 Oct 1;76(19):5907. doi: 10.1158/0008-5472.CAN-16-1853. Cancer Res. 2016. PMID: 27698190 No abstract available.
Abstract
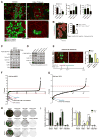
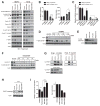
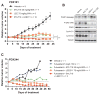
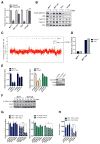
Small-molecule inhibitors of the CDK4/6 cell-cycle kinases have shown clinical efficacy in estrogen receptor (ER)-positive metastatic breast cancer, although their cytostatic effects are limited by primary and acquired resistance. Here we report that ER-positive breast cancer cells can adapt quickly to CDK4/6 inhibition and evade cytostasis, in part, via noncanonical cyclin D1-CDK2-mediated S-phase entry. This adaptation was prevented by cotreatment with hormone therapies or PI3K inhibitors, which reduced the levels of cyclin D1 (CCND1) and other G1-S cyclins, abolished pRb phosphorylation, and inhibited activation of S-phase transcriptional programs. Combined targeting of both CDK4/6 and PI3K triggered cancer cell apoptosis in vitro and in patient-derived tumor xenograft (PDX) models, resulting in tumor regression and improved disease control. Furthermore, a triple combination of endocrine therapy, CDK4/6, and PI3K inhibition was more effective than paired combinations, provoking rapid tumor regressions in a PDX model. Mechanistic investigations showed that acquired resistance to CDK4/6 inhibition resulted from bypass of cyclin D1-CDK4/6 dependency through selection of CCNE1 amplification or RB1 loss. Notably, although PI3K inhibitors could prevent resistance to CDK4/6 inhibitors, they failed to resensitize cells once resistance had been acquired. However, we found that cells acquiring resistance to CDK4/6 inhibitors due to CCNE1 amplification could be resensitized by targeting CDK2. Overall, our results illustrate convergent mechanisms of early adaptation and acquired resistance to CDK4/6 inhibitors that enable alternate means of S-phase entry, highlighting strategies to prevent the acquisition of therapeutic resistance to these agents. Cancer Res; 76(8); 2301-13. ©2016 AACR.
©2016 American Association for Cancer Research.
Conflict of interest statement
M. Bellet has received speakers’ bureau honoraria from Astra Zeneca. J. Cortes has received speakers’ bureau honoraria from Novartis. M. Dowsett reports receiving a commercial research grant from Pfizer and has received speakers’ bureau honoraria from Pfizer. L.-A. Martin reports receiving a commercial research grant from PUMA and Pfizer. N.C. Turner reports receiving a commercial research grant from Pfizer and Roche and is a consultant/advisory board member for Novartis, Pfizer, and Roche. No potential conflicts of interest were disclosed by the other authors.
Figures


References
-
- Yeo B, Turner NC, Jones A. An update on the medical management of breast cancer. BMJ. 2014;348:g3608. - PubMed
-
- Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52. - PubMed
-
- Yu Q, Geng Y, Sicinski P. Specific protection against breast cancers by cyclin D1 ablation. Nature. 2001;411:1017–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

