Efficacy of Artilysin Art-175 against Resistant and Persistent Acinetobacter baumannii
- PMID: 27021321
- PMCID: PMC4879360
- DOI: 10.1128/AAC.00285-16
Efficacy of Artilysin Art-175 against Resistant and Persistent Acinetobacter baumannii
Abstract
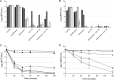
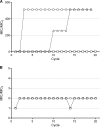
Bacteriophage-encoded endolysins have shown promise as a novel class of antibacterials with a unique mode of action, i.e., peptidoglycan degradation. However, Gram-negative pathogens are generally not susceptible due to their protective outer membrane. Artilysins overcome this barrier. Artilysins are optimized, engineered fusions of selected endolysins with specific outer membrane-destabilizing peptides. Artilysin Art-175 comprises a modified variant of endolysin KZ144 with an N-terminal fusion to SMAP-29. Previously, we have shown the high susceptibility of Pseudomonas aeruginosa to Art-175. Here, we report that Art-175 is highly bactericidal against stationary-phase cells of multidrug-resistant Acinetobacter baumannii, even resulting in a complete elimination of large inocula (≥10(8) CFU/ml). Besides actively dividing cells, Art-175 also kills persisters. Instantaneous killing of A. baumannii upon contact with Art-175 could be visualized after immobilization of the bacteria in a microfluidic flow cell. Effective killing of a cell takes place through osmotic lysis after peptidoglycan degradation. The killing rate is enhanced by the addition of 0.5 mM EDTA. No development of resistance to Art-175 under selection pressure and no cross-resistance with existing resistance mechanisms could be observed. In conclusion, Art-175 represents a highly active Artilysin against both A. baumannii and P. aeruginosa, two of the most life-threatening pathogens of the order Pseudomonadales.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures


References
-
- Valencia R, Arroyo LA, Conde M, Aldana JM, Torres M-J, Fernandez-Cuenca F, Garnacho-Montero J, Cisneros JM, Ortiz C, Pachon J, Aznar J. 2009. Nosocomial outbreak of infection with pan-drug-resistant Acinetobacter baumannii in a tertiary care university hospital. Infect Control Hosp Epidemiol 30:257–263. doi:10.1086/595977. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

