Pharmacological enhancement of mGlu5 receptors rescues behavioral deficits in SHANK3 knock-out mice
- PMID: 27021819
- PMCID: PMC5014121
- DOI: 10.1038/mp.2016.30
Pharmacological enhancement of mGlu5 receptors rescues behavioral deficits in SHANK3 knock-out mice
Erratum in
-
Pharmacological enhancement of mGlu5 receptors rescues behavioral deficits in SHANK3 knock-out mice.Mol Psychiatry. 2017 May;22(5):784. doi: 10.1038/mp.2016.70. Epub 2016 Apr 26. Mol Psychiatry. 2017. PMID: 27113996 No abstract available.
Abstract
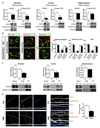
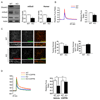
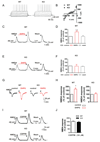
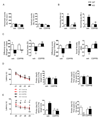
SHANK3 (also called PROSAP2) genetic haploinsufficiency is thought to be the major cause of neuropsychiatric symptoms in Phelan-McDermid syndrome (PMS). PMS is a rare genetic disorder that causes a severe form of intellectual disability (ID), expressive language delays and other autistic features. Furthermore, a significant number of SHANK3 mutations have been identified in patients with autism spectrum disorders (ASD), and SHANK3 truncating mutations are associated with moderate to profound ID. The Shank3 protein is a scaffold protein that is located in the postsynaptic density (PSD) of excitatory synapses and is crucial for synapse development and plasticity. In this study, we investigated the molecular mechanisms associated with the ASD-like behaviors observed in Shank3Δ11-/- mice, in which exon 11 has been deleted. Our results indicate that Shank3 is essential to mediating metabotropic glutamate receptor 5 (mGlu5)-receptor signaling by recruiting Homer1b/c to the PSD, specifically in the striatum and cortex. Moreover, augmenting mGlu5-receptor activity by administering 3-Cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamide ameliorated the functional and behavioral defects that were observed in Shank3Δ11-/- mice, suggesting that pharmaceutical treatments that increase mGlu5 activity may represent a new approach for treating patients that are affected by PMS and SHANK3 mutations.
Conflict of interest statement
Conflict of Interest
The authors declare they have no conflicts of interest.
Figures

References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

