Modeling ALS with motor neurons derived from human induced pluripotent stem cells
- PMID: 27021939
- PMCID: PMC5015775
- DOI: 10.1038/nn.4273
Modeling ALS with motor neurons derived from human induced pluripotent stem cells
Abstract
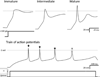
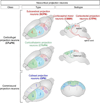
Directing the differentiation of induced pluripotent stem cells into motor neurons has allowed investigators to develop new models of amyotrophic lateral sclerosis (ALS). However, techniques vary between laboratories and the cells do not appear to mature into fully functional adult motor neurons. Here we discuss common developmental principles of both lower and upper motor neuron development that have led to specific derivation techniques. We then suggest how these motor neurons may be matured further either through direct expression or administration of specific factors or coculture approaches with other tissues. Ultimately, through a greater understanding of motor neuron biology, it will be possible to establish more reliable models of ALS. These in turn will have a greater chance of validating new drugs that may be effective for the disease.
Figures




References
-
- Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. - PubMed
-
- Yu J, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. - PubMed
-
- Sandoe J, Eggan K. Opportunities and challenges of pluripotent stem cell neurodegenerative disease models. Nature neuroscience. 2013;16:780–789. - PubMed
-
- Ericson J, Thor S, Edlund T, Jessell TM, Yamada T. Early stages of motor neuron differentiation revealed by expression of homeobox gene Islet-1. Science. 1992;256:1555–1560. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

