Regulation of Receptor for Advanced Glycation End Products (RAGE) Ectodomain Shedding and Its Role in Cell Function
- PMID: 27022018
- PMCID: PMC4933258
- DOI: 10.1074/jbc.M115.702399
Regulation of Receptor for Advanced Glycation End Products (RAGE) Ectodomain Shedding and Its Role in Cell Function
Abstract
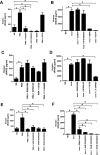
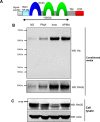
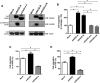
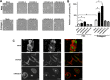
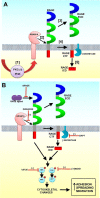
The receptor for advanced glycation end products (RAGE) is a multiligand transmembrane receptor that can undergo proteolysis at the cell surface to release a soluble ectodomain. Here we observed that ectodomain shedding of RAGE is critical for its role in regulating signaling and cellular function. Ectodomain shedding of both human and mouse RAGE was dependent on ADAM10 activity and induced with chemical activators of shedding (ionomycin, phorbol 12-myristate 13-acetate, and 4-aminophenylmercuric acetate) and endogenous stimuli (serum and RAGE ligands). Ectopic expression of the splice variant of RAGE (RAGE splice variant 4), which is resistant to ectodomain shedding, inhibited RAGE ligand dependent cell signaling, actin cytoskeleton reorganization, cell spreading, and cell migration. We found that blockade of RAGE ligand signaling with soluble RAGE or inhibitors of MAPK or PI3K blocked RAGE-dependent cell migration but did not affect RAGE splice variant 4 cell migration. We finally demonstrated that RAGE function is dependent on secretase activity as ADAM10 and γ-secretase inhibitors blocked RAGE ligand-mediated cell migration. Together, our data suggest that proteolysis of RAGE is critical to mediate signaling and cell function and may therefore emerge as a novel therapeutic target for RAGE-dependent disease states.
Keywords: ADAM; S100 proteins; cell migration; cell signaling; ectodomain shedding; matrix metalloproteinase (MMP); receptor for advanced glycation end products (RAGE).
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Kalea A. Z., Schmidt A. M., and Hudson B. I. (2009) RAGE: a novel biological and genetic marker for vascular disease. Clin. Sci. 116, 621–637 - PubMed
-
- Taguchi A., Blood D. C., del Toro G., Canet A., Lee D. C., Qu W., Tanji N., Lu Y., Lalla E., Fu C., Hofmann M. A., Kislinger T., Ingram M., Lu A., Tanaka H., et al. (2000) Blockage of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature 405, 354–360 - PubMed
-
- Hudson B. I., Kalea A. Z., Del Mar Arriero M., Harja E., Boulanger E., D'Agati V., and Schmidt A. M. (2008) Interaction of the RAGE cytoplasmic domain with diaphanous-1 is required for ligand-stimulated cellular migration through activation of Rac1 and Cdc42. J. Biol. Chem. 283, 34457–34468 - PMC - PubMed
-
- Logsdon C. D., Fuentes M. K., Huang E. H., and Arumugam T. (2007) RAGE and RAGE ligands in cancer. Curr. Mol. Med. 7, 777–789 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

