Cytotoxicity of atropine to human corneal endothelial cells by inducing mitochondrion-dependent apoptosis
- PMID: 27022135
- PMCID: PMC4994923
- DOI: 10.1177/1535370216640931
Cytotoxicity of atropine to human corneal endothelial cells by inducing mitochondrion-dependent apoptosis
Abstract
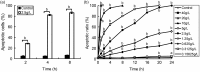
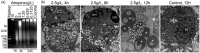
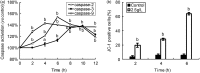
Atropine, a widely used topical anticholinergic drug, might have adverse effects on human corneas in vivo. However, its cytotoxic effect on human corneal endothelium (HCE) and its possible mechanisms are unclear. Here, we investigated the cytotoxicity of atropine and its underlying cellular and molecular mechanisms using an in vitro model of HCE cells and verified the cytotoxicity using cat corneal endothelium (CCE) in vivo. Our results showed that atropine at concentrations above 0.3125 g/L could induce abnormal morphology and viability decline in a dose- and time-dependent manner in vitro. The cytotoxicity of atropine was proven by the induced density decrease and abnormality of morphology and ultrastructure of CCE cells in vivo. Meanwhile, atropine could also induce dose- and time-dependent elevation of plasma membrane permeability, G1 phase arrest, phosphatidylserine externalization, DNA fragmentation, and apoptotic body formation of HCE cells. Moreover, 2.5 g/L atropine could also induce caspase-2/-3/-9 activation, mitochondrial transmembrane potential disruption, downregulation of anti-apoptotic Bcl-2 and Bcl-xL, upregulation of pro-apoptotic Bax and Bad, and upregulation of cytoplasmic cytochrome c and apoptosis-inducing factor. In conclusion, atropine above 1/128 of its clinical therapeutic dosage has a dose- and time-dependent cytotoxicity to HCE cells in vitro which is confirmed by CCE cells in vivo, and its cytotoxicity is achieved by inducing HCE cell apoptosis via a death receptor-mediated mitochondrion-dependent signaling pathway. Our findings provide new insights into the cytotoxicity and apoptosis-inducing effect of atropine which should be used with great caution in eye clinic.
Keywords: Atropine; apoptosis; cat corneal endothelial cells; cytotoxicity; human corneal endothelial cells; mitochondrion.
© 2016 by the Society for Experimental Biology and Medicine.
Figures




Similar articles
-
Cytotoxicity of atropine to human corneal epithelial cells by inducing cell cycle arrest and mitochondrion-dependent apoptosis.Exp Toxicol Pathol. 2015 Oct;67(10):517-24. doi: 10.1016/j.etp.2015.07.006. Epub 2015 Aug 19. Exp Toxicol Pathol. 2015. PMID: 26296992
-
The cytotoxic and pro-apoptotic effects of phenylephrine on corneal stromal cells via a mitochondrion-dependent pathway both in vitro and in vivo.Exp Toxicol Pathol. 2016 Aug;68(7):409-17. doi: 10.1016/j.etp.2016.06.003. Epub 2016 Jun 22. Exp Toxicol Pathol. 2016. PMID: 27344612
-
Cytotoxicity of proparacaine to human corneal endothelial cells in vitro.J Toxicol Sci. 2015 Aug;40(4):427-36. doi: 10.2131/jts.40.427. J Toxicol Sci. 2015. PMID: 26165639
-
Apoptotic effects of norfloxacin on corneal endothelial cells.Naunyn Schmiedebergs Arch Pharmacol. 2020 Jan;393(1):77-88. doi: 10.1007/s00210-019-01711-5. Epub 2019 Aug 16. Naunyn Schmiedebergs Arch Pharmacol. 2020. PMID: 31420720
-
Clonidine Induces Apoptosis of Human Corneal Epithelial Cells through Death Receptors-Mediated, Mitochondria-Dependent Signaling Pathway.Toxicol Sci. 2017 Mar 1;156(1):252-260. doi: 10.1093/toxsci/kfw249. Toxicol Sci. 2017. PMID: 28115640
Cited by
-
Establishing an Animal Model of Cytomegalovirus Keratouveitis in Rats: Broad Infection of Anterior Segment Tissue by Cytomegalovirus.Invest Ophthalmol Vis Sci. 2021 Oct 4;62(13):22. doi: 10.1167/iovs.62.13.22. Invest Ophthalmol Vis Sci. 2021. PMID: 34698772 Free PMC article.
-
Puerarin Attenuates LPS-Induced Inflammatory Responses and Oxidative Stress Injury in Human Umbilical Vein Endothelial Cells through Mitochondrial Quality Control.Oxid Med Cell Longev. 2021 Feb 27;2021:6659240. doi: 10.1155/2021/6659240. eCollection 2021. Oxid Med Cell Longev. 2021. Retraction in: Oxid Med Cell Longev. 2023 Oct 11;2023:9767123. doi: 10.1155/2023/9767123. PMID: 33728025 Free PMC article. Retracted.
-
Weak base drug-induced endolysosome iron dyshomeostasis controls the generation of reactive oxygen species, mitochondrial depolarization, and cytotoxicity.NeuroImmune Pharm Ther. 2024 Jan 11;3(1):33-46. doi: 10.1515/nipt-2023-0021. eCollection 2024 Mar. NeuroImmune Pharm Ther. 2024. PMID: 38532786 Free PMC article.
-
Morphological and Physiological Aspects of Mutable Collagenous Tissue at the Autotomy Plane of the Starfish Asterias rubens L. (Echinodermata, Asteroidea): An Echinoderm Paradigm.Mar Drugs. 2023 Feb 22;21(3):138. doi: 10.3390/md21030138. Mar Drugs. 2023. PMID: 36976186 Free PMC article. Review.
-
Rethinking the limitations of atropine in myopia control: new insights on binocular vision anomalies.Eye (Lond). 2025 Jul;39(10):1880-1881. doi: 10.1038/s41433-025-03864-5. Epub 2025 May 19. Eye (Lond). 2025. PMID: 40389560 No abstract available.
References
-
- Joyce NC. Proliferative capacity of the corneal endothelium. Prog Retin Eye Res 2003; 22: 359–89. - PubMed
-
- Hollingsworth J, Perez-Gomez I, Mutalib HA, Efron N. A population study of the normal cornea using an in vivo slit-scanning confocal microscope. Optom Vis Sci 2001; 78: 706–11. - PubMed
-
- Arnold RW, Gionet E, Hickel J, Owen M, Armitage MD. Duration and effect of single-dose atropine: paralysis of accommodation in penalization treatment of functional amblyopia. Binocul Vis Strabismus Q 2004; 19: 81–6. - PubMed
-
- Chia A, Chua WH, Cheung YB, Wong WL, Lingham A, Fong A, Tan D. Atropine for the treatment of childhood myopia: safety and efficacy of 0.5%, 0.1%, and 0.01% doses (Atropine for the Treatment of Myopia 2). Ophthalmology 2012; 19: 347–54. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

