Reverse vaccinology 2.0: Human immunology instructs vaccine antigen design
- PMID: 27022144
- PMCID: PMC4821650
- DOI: 10.1084/jem.20151960
Reverse vaccinology 2.0: Human immunology instructs vaccine antigen design
Abstract
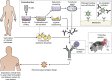
Traditionally, vaccines have been developed by cultivating infectious agents and isolating the inactivated whole pathogen or some of its purified components. 20 years ago, reverse vaccinology enabled vaccine discovery and design based on information deriving from the sequence of microbial genomes rather than via the growth of pathogens. Today, the high throughput discovery of protective human antibodies, sequencing of the B cell repertoire, and the increasing structural characterization of protective antigens and epitopes provide the molecular and mechanistic understanding to drive the discovery of novel vaccines that were previously impossible. We are entering a "reverse vaccinology 2.0" era.
© 2016 Rappuoli et al.
Figures
References
-
- Barouch D.H., Whitney J.B., Moldt B., Klein F., Oliveira T.Y., Liu J., Stephenson K.E., Chang H.W., Shekhar K., Gupta S., et al. . 2013. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature. 503:224–228. 10.1038/nature12744 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

