An evaluation of the safety and efficacy of bimatoprost for eyelash growth in pediatric subjects
- PMID: 27022239
- PMCID: PMC4792214
- DOI: 10.2147/OPTH.S89561
An evaluation of the safety and efficacy of bimatoprost for eyelash growth in pediatric subjects
Abstract
Purpose: Evaluate the safety and effectiveness of bimatoprost 0.03% for treatment of eyelash hypotrichosis in a pediatric population.
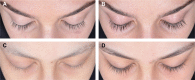
Patients and methods: This multicenter, randomized, double-masked, parallel-group study was conducted at seven sites in the US and Brazil. Subjects with eyelash hypotrichosis caused by chemotherapy or alopecia areata (aged 5-17 years) or healthy adolescents aged 15-17 years were enrolled (N=71). Subjects applied bimatoprost 0.03% or vehicle to upper eyelid margins once nightly for 4 months and were followed for 1 month post-treatment. Eyelash prominence was assessed using the validated 4-grade Global Eyelash Assessment scale with photonumeric guide. Changes in eyelash length, thickness, and darkness were measured by digital image analysis. Safety was assessed by adverse events and ophthalmic observations.
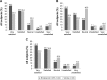
Results: Eyelash prominence improved in a significantly greater proportion of subjects treated with bimatoprost compared with vehicle at month 4 (70.8% versus 26.1%; P<0.001). This benefit was sustained at month 5 post-treatment assessment. Digital image analysis measures were significantly improved with bimatoprost. Significant treatment benefits with bimatoprost versus vehicle were evident among the healthy adolescents but not in the postchemotherapy or alopecia areata subgroups. The safety profile of bimatoprost was consistent with previous studies in adults.
Conclusion: Bimatoprost was safe and well tolerated in pediatric subjects with eyelash hypotrichosis. In this study with limited sample size, subgroup analyses showed that treatment was effective in healthy adolescents with no concurrent contributing medical condition, but not in those with eyelash hypotrichosis due to chemotherapy or alopecia areata.
Keywords: Latisse; adolescent; child; hypotrichosis.
Figures

Similar articles
-
Eyelash growth in subjects treated with bimatoprost: a multicenter, randomized, double-masked, vehicle-controlled, parallel-group study.J Am Acad Dermatol. 2012 May;66(5):801-6. doi: 10.1016/j.jaad.2011.06.005. Epub 2011 Sep 6. J Am Acad Dermatol. 2012. PMID: 21899919 Clinical Trial.
-
Bimatoprost for eyelash growth in Japanese subjects: two multicenter controlled studies.Aesthetic Plast Surg. 2014 Apr;38(2):451-60. doi: 10.1007/s00266-014-0293-7. Epub 2014 Mar 19. Aesthetic Plast Surg. 2014. PMID: 24643895 Free PMC article. Clinical Trial.
-
Safety and Efficacy of Bimatoprost for Eyelash Growth in Postchemotherapy Subjects.J Clin Aesthet Dermatol. 2015 Apr;8(4):11-20. J Clin Aesthet Dermatol. 2015. PMID: 26060513 Free PMC article.
-
Bimatoprost for the treatment of eyelash, eyebrow and scalp alopecia.Expert Opin Investig Drugs. 2017 Apr;26(4):515-522. doi: 10.1080/13543784.2017.1303480. Epub 2017 Mar 16. Expert Opin Investig Drugs. 2017. PMID: 28264599 Review.
-
Therapeutic potential of bimatoprost for the treatment of eyebrow hypotrichosis.Drug Des Devel Ther. 2018 Feb 22;12:365-372. doi: 10.2147/DDDT.S156467. eCollection 2018. Drug Des Devel Ther. 2018. PMID: 29503529 Free PMC article. Review.
Cited by
-
The efficacy of topical prostaglandin analogs for hair loss: A systematic review and meta-analysis.Front Med (Lausanne). 2023 Mar 14;10:1130623. doi: 10.3389/fmed.2023.1130623. eCollection 2023. Front Med (Lausanne). 2023. PMID: 36999072 Free PMC article. Review.
-
The physiological and pharmacological roles of prostaglandins in hair growth.Korean J Physiol Pharmacol. 2022 Nov 1;26(6):405-413. doi: 10.4196/kjpp.2022.26.6.405. Korean J Physiol Pharmacol. 2022. PMID: 36302616 Free PMC article. Review.
-
Bimatoprost versus Clobetasol Propionate in Scalp Alopecia Areata: A Prospective Non-Randomized Open-Label Clinical Trial.Indian Dermatol Online J. 2023 Mar 3;14(2):221-225. doi: 10.4103/idoj.idoj_299_22. eCollection 2023 Mar-Apr. Indian Dermatol Online J. 2023. PMID: 37089845 Free PMC article.
-
IADVL SIG Pediatric Dermatology (Academy) Recommendations on Childhood Alopecia Areata.Indian Dermatol Online J. 2022 Oct 31;13(6):710-720. doi: 10.4103/idoj.idoj_54_22. eCollection 2022 Nov-Dec. Indian Dermatol Online J. 2022. PMID: 36386742 Free PMC article. Review.
-
Treatment Options for Alopecia Areata in Children and Adolescents.Paediatr Drugs. 2024 May;26(3):245-257. doi: 10.1007/s40272-024-00620-2. Epub 2024 Mar 11. Paediatr Drugs. 2024. PMID: 38466519 Review.
References
-
- Kutner A, Friedman A. Hair loss in the dermatology office: an update on alopecia areata. J Drugs Dermatol. 2013;12:588–593. - PubMed
-
- Paus R, Haslam IS, Sharov AA, Botchkarev VA. Pathobiology of chemotherapy-induced hair loss. Lancet Oncol. 2013;14:e50–e59. - PubMed
-
- Draelos ZD. Special considerations in eye cosmetics. Clin Dermatol. 2001;19:424–430. - PubMed
-
- American Academy of Ophthalmology . American Academy of Ophthalmology warns consumers about the dangers of eyelash extensions [press release] San Francisco, CA: American Academy of Ophthalmology; [Accessed December 18, 2015]. Available from: http://www.aao.org/newsroom/news-releases/detail/american-academy-of-oph...
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

