Evaluating the role of atazanavir/cobicistat and darunavir/cobicistat fixed-dose combinations for the treatment of HIV-1 infection
- PMID: 27022304
- PMCID: PMC4790521
- DOI: 10.2147/HIV.S99063
Evaluating the role of atazanavir/cobicistat and darunavir/cobicistat fixed-dose combinations for the treatment of HIV-1 infection
Abstract
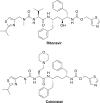
Atazanavir/cobicistat (ATV/c) and darunavir/cobicistat (DRV/c) are newly approved once daily fixed-dose protease inhibitor combinations for the treatment of HIV-1 infection. Studies in healthy volunteers have established bioequivalence between cobicistat and ritonavir as pharmacoenhancers of both atazanavir (ATV) and darunavir (DRV). In addition, two randomized clinical trials (one Phase II and one Phase III noninferiority trial with a 144-week followup period) demonstrated that cobicistat had sustainable and comparable efficacy and safety to ritonavir as a pharmacoenhancer of ATV through 144 weeks of treatment in HIV-1-infected patients. Furthermore, one Phase III, open-label, single-arm, clinical trial reflected virologic and immunologic responses and safety outcomes consistent with prior published data for DRV/ritonavir 800/100 mg once daily, supporting the use of DRV/c 800/150 mg once daily for future treatment of treatment-naïve and -experienced HIV-1-infected patients with no DRV resistance-associated mutations. Low rates of virologic failure secondary to resistance to antiretroviral regimens were present in these clinical studies. Most notable adverse events in the ATV studies were hyperbilirubinemia and in the DRV study rash. Small increases in serum creatinine and minimally reduced estimated glomerular filtration rate Cockcroft-Gault calculation (eGFRCG) were observed in ATV/c and DRV/c clinical studies consistent with other studies evaluating elvitegravir/cobicistat/tenofovir/emtricitabine for the treatment of HIV-1 infection. These renal parameter changes occurred acutely in the first few weeks and plateaued off for the remaining study periods and are not necessarily clinically relevant. Cobicistat has numerous advantages compared to ritonavir such as fewer drug-drug interactions, being devoid of anti-HIV-1 activity, as well as it has better solubility affording coformulation with other antiretrovirals as simplified fixed-dose combinations. Overall, the recent approval of ATV/c and DRV/c offers HIV patients opportunities for improved adherence to lifelong treatment. Future studies are warranted to determine the efficacy and safety of ATV/c and DRV/c in treatment-experienced patients.
Keywords: HIV protease inhibitors; atazanavir; cobicistat; darunavir; treatment simplification.
Figures
Similar articles
-
Phase 2 study of cobicistat versus ritonavir each with once-daily atazanavir and fixed-dose emtricitabine/tenofovir df in the initial treatment of HIV infection.AIDS. 2011 Sep 24;25(15):1881-6. doi: 10.1097/QAD.0b013e32834b4d48. AIDS. 2011. PMID: 21811136 Clinical Trial.
-
Metabolic effects of darunavir/ritonavir versus atazanavir/ritonavir in treatment-naive, HIV type 1-infected subjects over 48 weeks.AIDS Res Hum Retroviruses. 2012 Oct;28(10):1184-95. doi: 10.1089/aid.2011.0327. Epub 2012 Apr 2. AIDS Res Hum Retroviruses. 2012. PMID: 22352336 Free PMC article. Clinical Trial.
-
Darunavir-cobicistat-emtricitabine-tenofovir alafenamide: safety and efficacy of a protease inhibitor in the modern era.Drug Des Devel Ther. 2018 Oct 29;12:3635-3643. doi: 10.2147/DDDT.S147493. eCollection 2018. Drug Des Devel Ther. 2018. PMID: 30464395 Free PMC article. Review.
-
Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate versus ritonavir-boosted atazanavir plus co-formulated emtricitabine and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3, non-inferiority trial.Lancet. 2012 Jun 30;379(9835):2429-2438. doi: 10.1016/S0140-6736(12)60918-0. Lancet. 2012. PMID: 22748590 Clinical Trial.
-
Atazanavir sulfate + cobicistat for the treatment of HIV infection.Expert Rev Anti Infect Ther. 2017 Jun;15(6):569-576. doi: 10.1080/14787210.2017.1323634. Epub 2017 May 9. Expert Rev Anti Infect Ther. 2017. PMID: 28443391 Review.
Cited by
-
Therapeutic Potential of Spirooxindoles as Antiviral Agents.ACS Infect Dis. 2016 Jun 10;2(6):382-92. doi: 10.1021/acsinfecdis.6b00041. Epub 2016 May 5. ACS Infect Dis. 2016. PMID: 27627626 Free PMC article. Review.
-
A clinical review of HIV integrase strand transfer inhibitors (INSTIs) for the prevention and treatment of HIV-1 infection.Retrovirology. 2022 Oct 22;19(1):22. doi: 10.1186/s12977-022-00608-1. Retrovirology. 2022. PMID: 36273165 Free PMC article. Review.
-
An Overview of Human Immunodeficiency Virus-1 Antiretroviral Drugs: General Principles and Current Status.Infect Chemother. 2021 Mar;53(1):29-45. doi: 10.3947/ic.2020.0100. Infect Chemother. 2021. PMID: 34409780 Free PMC article. Review.
References
-
- Department of Health and Human Services Guidelines for the use of antiretroviral agents in HIV-1 infected adults and adolescents. Apr, 2015. [Accessed September 16, 2015]. Available from: https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-treatmen....
-
- Williams I, Churchill D, Anderson J, et al. British HIV Association guidelines for the treatment of HIV-1-positive adults with antiretroviral therapy 2015. HIV Med; 2015. [Accessed October 21, 2015]. Available from: http://www.bhiva.org/documents/Guidelines/Treatment/2015/2015-treatment-.... - PubMed
-
- Arya V, Robertson SM, Struble KA, Murray JS. Scientific considerations for pharmacoenhancers in antiretroviral therapy. J Clin Pharmacol. 2012;52(8):1128–1133. - PubMed
-
- Josephson F. Drug-drug interactions in the treatment of HIV infection: focus on pharmacokinetic enhancement through CYP3A inhibition. J Intern Med. 2010;268(6):530–539. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

