Fibrin sealant use in pilonidal sinus: Systematic review
- PMID: 27022454
- PMCID: PMC4807328
- DOI: 10.4240/wjgs.v8.i3.266
Fibrin sealant use in pilonidal sinus: Systematic review
Abstract
Aim: To review the current data about the success rates of fibrin sealant use in pilonidal disease.
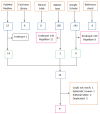
Methods: Fibrin sealant can be used for different purposes in pilonidal sinus treatment, such as filling in the sinus tracts, covering the open wound after excision and lay-open treatment, or obliterating the subcutaneous dead space before skin closure. We searched Pubmed, Google-Scholar, Ebsco-Host, clinicaltrials, and Cochrane databases and found nine studies eligible for analysis; these studies included a total of 217 patients (84% male, mean age 24.2 ± 7.8).
Results: In cases where fibrin sealant was used to obliterate the subcutaneous dead space, there was no reduction in wound complication rates (9.8% vs 14.6%, P = 0.48). In cases where sealant was used to cover the laid-open area, the wound healing time and patient comfort were reported better than in previous studies (mean 17 d, 88% satisfaction). When fibrin sealant was used to fill the sinus tracts, the recurrence rate was around 20%, despite the highly selected grouping of patients.
Conclusion: Consequently, using fibrin sealant to decrease the risk of seroma formation was determined to be an ineffective course of action. It was not advisable to fill the sinus tracts with fibrin sealant because it was not superior to other cost-effective and minimally invasive treatments. New comparative studies can be conducted to confirm the results of sealant use in covering the laid-open area.
Keywords: Evidence base medicine; Fibrin sealant; Pilonidal disease; Systematic review.
Figures
Comment in
-
The Author Replies.Dis Colon Rectum. 2017 May;60(5):e32-e33. doi: 10.1097/DCR.0000000000000813. Dis Colon Rectum. 2017. PMID: 28383458 No abstract available.
References
-
- Hull TL, Wu J. Pilonidal disease. Surg Clin North Am. 2002;82:1169–1185. - PubMed
-
- Kayaalp C. Basic calculating errors in systematic reviews. Obes Surg. 2013;23:1673. - PubMed
-
- Greenberg R, Kashtan H, Skornik Y, Werbin N. Treatment of pilonidal sinus disease using fibrin glue as a sealant. Tech Coloproctol. 2004;8:95–98. - PubMed
-
- Lund JN, Leveson SH. Fibrin glue in the treatment of pilonidal sinus: results of a pilot study. Dis Colon Rectum. 2005;48:1094–1096. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources