Post-operative abdominal complications in Crohn's disease in the biological era: Systematic review and meta-analysis
- PMID: 27022455
- PMCID: PMC4807329
- DOI: 10.4240/wjgs.v8.i3.274
Post-operative abdominal complications in Crohn's disease in the biological era: Systematic review and meta-analysis
Abstract
Aim: To perform a systematic review and meta-analysis on post-operative complications after surgery for Crohn's disease (CD) comparing biological with no therapy.
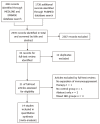
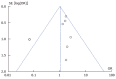
Methods: PubMed, Medline and Embase databases were searched to identify studies comparing post-operative outcomes in CD patients receiving biological therapy and those who did not. A meta-analysis with a random-effects model was used to calculate pooled odds ratios (OR) and confidence intervals (CI) for each outcome measure of interest.
Results: A total of 14 studies were included for meta-analysis, comprising a total of 5425 patients with CD 1024 (biological treatment, 4401 control group). After biological therapy there was an increased risk of total infectious complications (OR = 1.52; 95%CI: 1.14-2.03, 8 studies) and wound infection (OR = 1.73; 95%CI: 1.12-2.67; P = 0.01, 7 studies). There was no increased risk for other complications including anastomotic leak (OR = 1.19; 95%CI: 0.82-1.71; P = 0.26), abdominal sepsis (OR = 1.22; 95%CI: 0.87-1.72; P = 0.25) and re-operation (OR = 1.12; 95%CI: 0.81-1.54; P = 0.46) in patients receiving biological therapy.
Conclusion: Pre-operative use of anti-TNF-α therapy may increase risk of post-operative infectious complications after surgery for CD and in particular wound related infections.
Keywords: Adulimimab; Anti-tumor necrosis factor-α; Biological; Crohn’s; Infliximab; Monoclonal antibody; Post-operative complications.
Figures





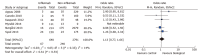
References
-
- Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, Wolf DC, Olson A, Bao W, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–1549. - PubMed
-
- Feagan BG, Panaccione R, Sandborn WJ, D’Haens GR, Schreiber S, Rutgeerts PJ, Loftus EV, Lomax KG, Yu AP, Wu EQ, et al. Effects of adalimumab therapy on incidence of hospitalization and surgery in Crohn’s disease: results from the CHARM study. Gastroenterology. 2008;135:1493–1499. - PubMed
-
- D’Haens GR, Panaccione R, Higgins PD, Vermeire S, Gassull M, Chowers Y, Hanauer SB, Herfarth H, Hommes DW, Kamm M, et al. The London Position Statement of the World Congress of Gastroenterology on Biological Therapy for IBD with the European Crohn’s and Colitis Organization: when to start, when to stop, which drug to choose, and how to predict response? Am J Gastroenterol. 2011;106:199–212; quiz 213. - PubMed
-
- Peyrin-Biroulet L, Desreumaux P, Sandborn WJ, Colombel JF. Crohn’s disease: beyond antagonists of tumour necrosis factor. Lancet. 2008;372:67–81. - PubMed
-
- Billioud V, Sandborn WJ, Peyrin-Biroulet L. Loss of response and need for adalimumab dose intensification in Crohn’s disease: a systematic review. Am J Gastroenterol. 2011;106:674–684. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

