Clearance of rapid adenosine release is regulated by nucleoside transporters and metabolism
- PMID: 27022463
- PMCID: PMC4777247
- DOI: 10.1002/prp2.189
Clearance of rapid adenosine release is regulated by nucleoside transporters and metabolism
Abstract
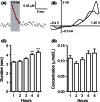
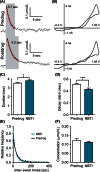
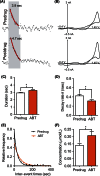
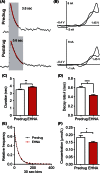
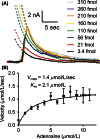
Adenosine is a neuromodulator that regulates neurotransmission in the brain and central nervous system. Recently, spontaneous adenosine release that is cleared in 3-4 sec was discovered in mouse spinal cord slices and anesthetized rat brains. Here, we examined the clearance of spontaneous adenosine in the rat caudate-putamen and exogenously applied adenosine in caudate brain slices. The V max for clearance of exogenously applied adenosine in brain slices was 1.4 ± 0.1 μmol/L/sec. In vivo, the equilibrative nucleoside transport 1 (ENT1) inhibitor, S-(4-nitrobenzyl)-6-thioinosine (NBTI) (1 mg/kg, i.p.) significantly increased the duration of adenosine, while the ENT1/2 inhibitor, dipyridamole (10 mg/kg, i.p.), did not affect duration. 5-(3-Bromophenyl)-7-[6-(4-morpholinyl)-3-pyrido[2,3-d]byrimidin-4-amine dihydrochloride (ABT-702), an adenosine kinase inhibitor (5 mg/kg, i.p.), increased the duration of spontaneous adenosine release. The adenosine deaminase inhibitor, erythro-9-(2-hydroxy-3-nonyl)adenine (EHNA) (10 mg/kg, i.p.), also increased the duration in vivo. Similarly, NBTI (10 μmol/L), ABT-702 (100 nmol/L), or EHNA (20 μmol/L) also decreased the clearance rate of exogenously applied adenosine in brain slices. The increases in duration for blocking ENT1, adenosine kinase, or adenosine deaminase individually were similar, about 0.4 sec in vivo; thus, the removal of adenosine on a rapid time scale occurs through three mechanisms that have comparable effects. A cocktail of ABT-702, NBTI, and EHNA significantly increased the duration by 0.7 sec, so the mechanisms are not additive and there may be additional mechanisms clearing adenosine on a rapid time scale. The presence of multiple mechanisms for adenosine clearance on a time scale of seconds demonstrates that adenosine is tightly regulated in the extracellular space.
Keywords: Adenosine deaminase; adenosine kinase; equilibrative nucleoside transporter; voltammetry.
Figures


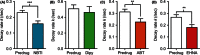
References
-
- Anderson CM, Sitar DS, Parkinson FE (1996). Ability of nitrobenzylthioinosine to cross the blood‐brain barrier in rats. Neurosci Lett 219: 191–194. - PubMed
-
- Anderson CM, Xiong W, Geiger JD, Young JD, Cass CE, Baldwin SA, et al. (1999). Distribution of equilibrative, nitrobenzylthioinosine‐sensitive nucleoside transporters (ENT1) in brain. J Neurochem 73: 867–873. - PubMed
-
- Bailey A, Weber D, Zimmer A, Zimmer AM, Hourani SM, Kitchen I (2004). Quantitative autoradiography of adenosine receptors and NBTI‐sensitive adenosine transporters in the brains of mice deficient in the preproenkephalin gene. Brain Res 1025: 1–9. - PubMed
-
- Baldwin SA, Yao SYM, Hyde RJ, Ng AML, Foppolo S, Barnes K, et al. (2005). Functional characterization of novel human and mouse equilibrative nucleoside transporters (hENT3 and mENT3) located in intracellular membranes. J Biol Chem 280: 15880–15887. - PubMed
-
- Ballarin M, Fredholm BB, Ambrosio S, Mahy N (1991). Extracellular levels of adenosine and its metabolites in the striatum of awake rats: inhibition of uptake and metabolism. Acta Physiol Scand 142: 97–103. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

