Preclinical pharmacology and pharmacokinetics of CERC-301, a GluN2B-selective N-methyl-D-aspartate receptor antagonist
- PMID: 27022470
- PMCID: PMC4777252
- DOI: 10.1002/prp2.198
Preclinical pharmacology and pharmacokinetics of CERC-301, a GluN2B-selective N-methyl-D-aspartate receptor antagonist
Abstract
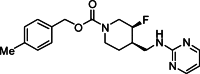
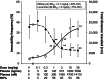
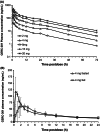
The preclinical pharmacodynamic and pharmacokinetic properties of 4-methylbenzyl (3S, 4R)-3-fluoro-4-[(Pyrimidin-2-ylamino) methyl] piperidine-1-carboxylate (CERC-301), an orally bioavailable selective N-methyl-D-aspartate (NMDA) receptor subunit 2B (GluN2B) antagonist, were characterized to develop a translational approach based on receptor occupancy (RO) to guide CERC-301 dose selection in clinical trials of major depressive disorder. CERC-301 demonstrated high-binding affinity (K i, 8.1 nmol L(-1)) specific to GluN2B with an IC 50 of 3.6 nmol L(-1) and no off-target activity. CERC-301 efficacy was demonstrated in the forced swim test with an efficacy dose (ED 50) of 0.3-0.7 mg kg(-1) (RO, 30-50%); increase in locomotor activity was observed at ED 50 of 2 mg kg(-1), corresponding to an RO of 75%. The predicted 50% RO concentration (Occ50) in humans was 400 nmol L(-1), similar to that predicted for rat, dog, and monkey (300, 200, and 400 nmol L(-1), respectively). Safety pharmacology and neurotoxicity studies raised no specific safety concerns. A first-in-human study in healthy males demonstrated a dose-proportional pharmacokinetic profile, with T max of ~1 h and t 1/2 of 12-17 h. Based on the preclinical and pharmacodynamic data, doses of ≥8 mg in humans are hypothesized to have an acceptable safety profile and result in clinically relevant peak plasma exposure.
Keywords: Depression; GluN2B; NMDA antagonist; major depressive disorder.
Figures


References
-
- Addy C, Assaid C, Hreniuk D, Stroh M, Xu Y, Herring WJ, et al. (2009). Single‐dose administration of MK‐0657, an NR2B‐selective NMDA antagonist, does not result in clinically meaningful improvement in motor function in patients with moderate Parkinson's disease. J Clin Pharmacol 49: 856–864. - PubMed
-
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. (2000). Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47: 351–354. - PubMed
-
- Bhatt JM, Prakash A, Suryavanshi PS, Dravid SM (2013). Effect of ifenprodil on GluN1/GluN2B N‐Methyl‐D‐aspartate receptor gating. Mol Pharmacol 83: 9–21. - PubMed
-
- Bresink I, Danysz W, Parsons CG, Mutschler E (1995). Different binding affinities of NMDA receptor channel blockers in various brain regions–indication of NMDA receptor heterogeneity. Neuropharmacology 34: 533–540. - PubMed
-
- Claiborne CF, Liverton NJ, Libby B, Curtis NR, Kulagowski J (2000). Aryl amidines as NMDA NR2B antagonists. PCT Int Appl: WO2000067751.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

