De Novo-Synthesized Retinoic Acid in Ovarian Antral Follicles Enhances FSH-Mediated Ovarian Follicular Cell Differentiation and Female Fertility
- PMID: 27022678
- PMCID: PMC4870881
- DOI: 10.1210/en.2015-2064
De Novo-Synthesized Retinoic Acid in Ovarian Antral Follicles Enhances FSH-Mediated Ovarian Follicular Cell Differentiation and Female Fertility
Abstract
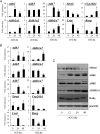
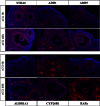
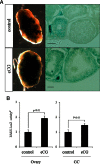
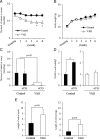
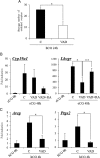
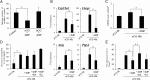
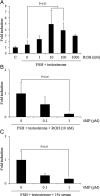
Retinoic acid (RA) is the active form of vitamin A and is synthesized from retinol by two key enzymes, alcohol dehydrogenase (ADH) and acetaldehyde dehydrogenase (ALDH). As the physiological precursor of RA, retinol impacts female reproductive functions and fertility. The expression of Adh1 and Adh5 as well as Aldh1a1 and Aldh1a7 are significantly increased in the ovaries of mice treated with equine chorionic gonadotropin/FSH. The RA receptor is expressed and localized in granulosa cells and is activated by endogenous RA as indicated by LacZ expression in granulosa cells of RA-responsive transgene-LacZ transgenic mice (RA reporter mice). Coinjection of the ADH inhibitor, 4-methylpyrazole, with equine chorionic gonadotropin significantly decreases the number and developmental competence of oocytes ovulated in response to human chorionic gonadotropin/LH as compared with controls. Injections of RA completely reverse the effects of the inhibitor of ovulation and oocyte development. When mice were fed a retinol-free, vitamin A-deficient diet that significantly reduced the serum levels of retinol, the expression of the LH receptor (Lhcgr) was significantly lower in the ovaries of the vitamin A-deficient mice, and injections of human chorionic gonadotropin failed to induce genes controlling ovulation. These results indicate that ovarian de novo biosynthesis of RA is required for the follicular expression of Lhcgr in granulosa cells and their ability to respond to the ovulatory LH surge.
Figures
References
-
- Richards JS. Hormonal control of gene expression in the ovary. Endocr Rev. 1994;15:725–751. - PubMed
-
- Elvin JA, Yan C, Matzuk MM. Oocyte-expressed TGF-β superfamily members in female fertility. Mol Cell Endocrinol. 2000;159:1–5. - PubMed
-
- Shimasaki S, Moore RK, Otsuka F, Erickson GF. The bone morphogenetic protein system in mammalian reproduction. Endocr Rev. 2004;25:72–101. - PubMed
-
- Barnett KR, Schilling C, Greenfeld CR, Tomic D, Flaws JA. Ovarian follicle development and transgenic mouse models. Hum Reprod. 2006;12:537–555. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

