Gap Junctions Contribute to the Regulation of Walking-Like Activity in the Adult Mudpuppy (Necturus Maculatus)
- PMID: 27023006
- PMCID: PMC4811563
- DOI: 10.1371/journal.pone.0152650
Gap Junctions Contribute to the Regulation of Walking-Like Activity in the Adult Mudpuppy (Necturus Maculatus)
Abstract
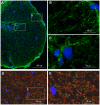
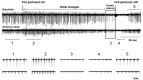
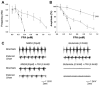
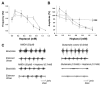
Although gap junctions are widely expressed in the developing central nervous system, the role of electrical coupling of neurons and glial cells via gap junctions in the spinal cord in adults is largely unknown. We investigated whether gap junctions are expressed in the mature spinal cord of the mudpuppy and tested the effects of applying gap junction blocker on the walking-like activity induced by NMDA or glutamate in an in vitro mudpuppy preparation. We found that glial and neural cells in the mudpuppy spinal cord expressed different types of connexins that include connexin 32 (Cx32), connexin 36 (Cx36), connexin 37 (Cx37), and connexin 43 (Cx43). Application of a battery of gap junction blockers from three different structural classes (carbenexolone, flufenamic acid, and long chain alcohols) substantially and consistently altered the locomotor-like activity in a dose-dependent manner. In contrast, these blockers did not significantly change the amplitude of the dorsal root reflex, indicating that gap junction blockers did not inhibit neuronal excitability nonselectively in the spinal cord. Taken together, these results suggest that gap junctions play a significant modulatory role in the spinal neural networks responsible for the generation of walking-like activity in the adult mudpuppy.
Conflict of interest statement
Figures

Similar articles
-
Immunogold evidence that neuronal gap junctions in adult rat brain and spinal cord contain connexin-36 but not connexin-32 or connexin-43.Proc Natl Acad Sci U S A. 2000 Jun 20;97(13):7573-8. doi: 10.1073/pnas.97.13.7573. Proc Natl Acad Sci U S A. 2000. PMID: 10861019 Free PMC article.
-
Differential distribution of interneurons in the neural networks that control walking in the mudpuppy (Necturus maculatus) spinal cord.Exp Brain Res. 2002 Jul;145(2):190-8. doi: 10.1007/s00221-002-1102-0. Epub 2002 May 9. Exp Brain Res. 2002. PMID: 12110959
-
Re-evaluation of connexins associated with motoneurons in rodent spinal cord, sexually dimorphic motor nuclei and trigeminal motor nucleus.Eur J Neurosci. 2014 Mar;39(5):757-70. doi: 10.1111/ejn.12450. Epub 2013 Dec 9. Eur J Neurosci. 2014. PMID: 24313680 Free PMC article.
-
Role of connexins in spinal cord injury: An update.Clin Neurol Neurosurg. 2020 Oct;197:106102. doi: 10.1016/j.clineuro.2020.106102. Epub 2020 Jul 21. Clin Neurol Neurosurg. 2020. PMID: 32717564 Review.
-
Update on connexins and gap junctions in neurons and glia in the mammalian nervous system.Brain Res Brain Res Rev. 2004 Dec;47(1-3):191-215. doi: 10.1016/j.brainresrev.2004.05.005. Brain Res Brain Res Rev. 2004. PMID: 15572172 Review.
Cited by
-
Combined Supra- and Sub-Lesional Epidural Electrical Stimulation for Restoration of the Motor Functions after Spinal Cord Injury in Mini Pigs.Brain Sci. 2020 Oct 16;10(10):744. doi: 10.3390/brainsci10100744. Brain Sci. 2020. PMID: 33081405 Free PMC article.
-
Epidural Stimulation Combined with Triple Gene Therapy for Spinal Cord Injury Treatment.Int J Mol Sci. 2020 Nov 24;21(23):8896. doi: 10.3390/ijms21238896. Int J Mol Sci. 2020. PMID: 33255323 Free PMC article.
-
Chronic Stress and Gonadectomy Affect the Expression of Cx37, Cx40 and Cx43 in the Spinal Cord.Life (Basel). 2021 Dec 1;11(12):1330. doi: 10.3390/life11121330. Life (Basel). 2021. PMID: 34947861 Free PMC article.
References
-
- Feller MB. Spontaneous correlated activity in developing neural circuits. Neuron 1999. 22:653–656. - PubMed
-
- Sutor B, Hagerty T. Involvement of gap junctions in the development of the neocortex. Biochim Biophys Acta 2005. 1719:59–68. - PubMed
-
- Bennett MV. Gap junctions as electrical synapses. J Neurocytol 1997. 26:349–366. - PubMed
-
- Tamas G, Buhl EH, Lorincz A, Somogyi P. Proximally targeted GABAergic synapses and gap junctions synchronize cortical interneurons. Nat Neurosci 2000. 3:366–371. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

