Deletion of JMJD2B in neurons leads to defective spine maturation, hyperactive behavior and memory deficits in mouse
- PMID: 27023172
- PMCID: PMC4872455
- DOI: 10.1038/tp.2016.31
Deletion of JMJD2B in neurons leads to defective spine maturation, hyperactive behavior and memory deficits in mouse
Abstract
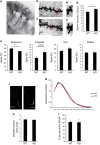
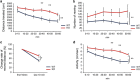
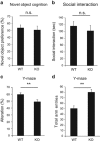
JMJD2B is a histone demethylase enzyme that regulates gene expression through demethylation of H3K9me3. Although mutations of JMJD2B have been suggested to be responsible for neurodevelopmental disorders, the function of JMJD2B in the central nervous system (CNS) remains to be elucidated. Here we show that JMJD2B has a critical role in the development of the CNS. We observed JMJD2B expression, which was especially strong in the hippocampus, throughout the CNS from embryonic periods through adulthood. We generated neuron-specific JMJD2B-deficient mice using the cre-loxP system. We found an increase in total spine number, but a decrease in mature spines, in the CA1 region of the hippocampus. JMJD2B-deficient mice exhibited hyperactive behavior, sustained hyperactivity in a novel environment, deficits in working memory and spontaneous epileptic-like seizures. Together these observations indicate that JMJD2B mutant mice display symptoms reminiscent of neurodevelopmental disorders. Our findings provide evidence for the involvement of histone demethylation in the formation of functional neural networks during development.
Figures


Similar articles
-
Anterior thalamic lesions reduce spine density in both hippocampal CA1 and retrosplenial cortex, but enrichment rescues CA1 spines only.Hippocampus. 2014 Oct;24(10):1232-47. doi: 10.1002/hipo.22309. Epub 2014 Jun 13. Hippocampus. 2014. PMID: 24862603
-
Loss of X-linked mental retardation gene oligophrenin1 in mice impairs spatial memory and leads to ventricular enlargement and dendritic spine immaturity.J Neurosci. 2007 Aug 29;27(35):9439-50. doi: 10.1523/JNEUROSCI.2029-07.2007. J Neurosci. 2007. PMID: 17728457 Free PMC article.
-
Aberrant hippocampal spine morphology and impaired memory formation in neuronal platelet-derived growth factor β-receptor lacking mice.Hippocampus. 2012 Jun;22(6):1371-8. doi: 10.1002/hipo.20973. Epub 2011 Oct 13. Hippocampus. 2012. PMID: 21997856
-
Mutant genetic background affects the functional rearrangement and kinetic properties of JMJD2b histone demethylase.J Mol Biol. 2011 Jan 21;405(3):679-95. doi: 10.1016/j.jmb.2010.11.001. Epub 2010 Nov 10. J Mol Biol. 2011. PMID: 21073875
-
Histone demethylase JMJD2B is required for tumor cell proliferation and survival and is overexpressed in gastric cancer.Biochem Biophys Res Commun. 2011 Dec 16;416(3-4):372-8. doi: 10.1016/j.bbrc.2011.11.045. Epub 2011 Nov 19. Biochem Biophys Res Commun. 2011. PMID: 22133676
Cited by
-
Epigenetic Changes in Alzheimer's Disease: DNA Methylation and Histone Modification.Cells. 2024 Apr 21;13(8):719. doi: 10.3390/cells13080719. Cells. 2024. PMID: 38667333 Free PMC article. Review.
-
Genotype-phenotype correlation study of structural abnormalities in a fetal brain caused by a novel KDM4B variant.Mol Biol Rep. 2024 Jan 25;51(1):188. doi: 10.1007/s11033-023-09092-y. Mol Biol Rep. 2024. PMID: 38270710
-
Targeted Downregulation of kdm4a Ameliorates Tau-engendered Defects in Drosophila melanogaster.J Korean Med Sci. 2019 Aug 26;34(33):e225. doi: 10.3346/jkms.2019.34.e225. J Korean Med Sci. 2019. PMID: 31436053 Free PMC article.
-
Accelerated Deficits of Spatial Learning and Memory Resulting From Prenatal Inflammatory Insult Are Correlated With Abnormal Phosphorylation and Methylation of Histone 3 in CD-1 Mice.Front Aging Neurosci. 2019 May 16;11:114. doi: 10.3389/fnagi.2019.00114. eCollection 2019. Front Aging Neurosci. 2019. PMID: 31156421 Free PMC article.
-
Heterozygous Variants in KDM4B Lead to Global Developmental Delay and Neuroanatomical Defects.Am J Hum Genet. 2020 Dec 3;107(6):1170-1177. doi: 10.1016/j.ajhg.2020.11.001. Epub 2020 Nov 23. Am J Hum Genet. 2020. PMID: 33232677 Free PMC article.
References
-
- Tuchman R, Cuccaro M. Epilepsy and autism: neurodevelopmental perspective. Curr Neurol Neurosci Rep 2011; 11: 428–443 4. - PubMed
-
- Gathercole SE, Alloway TP. Practitioner review: short-term and working memory impairments in neurodevelopmental disorders: diagnosis and remedial support. J Child Psychol Psychiatry 2006; 47: 4–15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

