Optical Coherence Tomography Angiography of Dry Age-Related Macular Degeneration
- PMID: 27023214
- PMCID: PMC5875686
- DOI: 10.1159/000442784
Optical Coherence Tomography Angiography of Dry Age-Related Macular Degeneration
Abstract
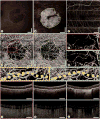
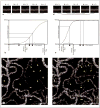
Optical coherence tomography angiography (OCTA) can be used to visualize alterations in the choriocapillaris of patients with dry age-related macular degeneration (AMD). These changes seem to be present during all stages of the disease. Earlier stages are associated with patchy thinning of the choriocapillaris, while geographic atrophy is associated with loss of choriocapillaris lying under the area of geographic atrophy and asymmetric alteration of choriocapillaris at the margins of the geographic atrophy. The use of high-speed, long-wave-length swept-source OCT for angiography, with its better penetration into the choroid and high acquisition speeds, enable OCTA with scaled slowest detectable flow and fastest distinguishable flow. This will enable us to better investigate choriocapillaris changes in patients with dry AMD. The ability to image the choriocapillaris structure and flow impairments may be useful in the future for detecting and monitoring the progression of dry AMD and for monitoring treatment responses in clinical trials to therapies that target disease progression in dry AMD.
© 2016 S. Karger AG, Basel.
Figures

References
-
- Velez-Montoya R, Oliver SC, Olson JL, Fine SL, Quiroz-Mercado H, Mandava N. Current knowledge and trends in age-related macular degeneration: genetics, epidemiology, and prevention. Retina. 2014;34:423–441. - PubMed
-
- Friedman DS, OColmain BJ, Munoz B, Tomany SC, McCarty C, de Jong PT, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572. - PubMed
-
- Fleckenstein M, Schmitz-Valckenberg S, Adrion C, Kramer I, Eter N, Helb HM, et al. Tracking progression with spectral-domain optical coherence tomography in geographic atrophy caused by age-related macular degeneration. Invest Ophthalmol Vis Sci. 2010;51:3846–3852. - PubMed
-
- Nunes RP, Gregori G, Yehoshua Z, Stetson PF, Feuer W, Moshfeghi AA, et al. Predicting the progression of geographic atrophy in age-related macular degeneration with SD-OCT en face imaging of the outer retina. Ophthalmic Surg Lasers Imaging Retina. 2013;44:344–359. - PubMed
-
- Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. 1992;99:933–943. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

